Figures & data
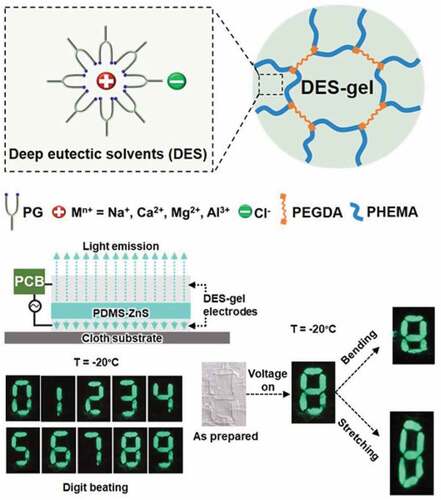
Figure 1. (a) Schematic illustration of DES molecular structure. (b) Viscosity of DES as a function of the concentration of MCln.
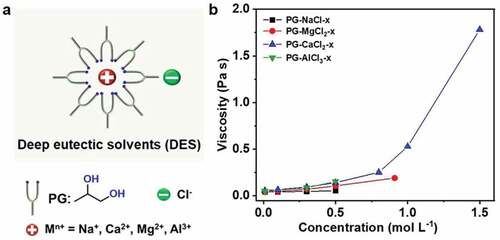
Figure 2. (a) Preparation of DES-gels based on DES. Photos of [PG-CaCl2-0.5]-gel at (b) 20°C and (c) −60°C. The as-prepared gel possesses high optical transparency and flexibility over a wide temperature range
![Figure 2. (a) Preparation of DES-gels based on DES. Photos of [PG-CaCl2-0.5]-gel at (b) 20°C and (c) −60°C. The as-prepared gel possesses high optical transparency and flexibility over a wide temperature range](/cms/asset/f507db6d-e696-439b-a98e-d8c24f718ec6/tsnm_a_1972053_f0002_c.jpg)
Figure 3. (a) Conductivity of various DES-gels at different concentration of MCln. In certain range of concentrations, the conductivity becomes much larger under a higher salt concentration. However, the conductivity shows a downward trend when the concentration of salt increases further. (b) Conductivity of [PG-CaCl2-0.5]-gel at different temperatures. Conductivity increases as the temperature increases
![Figure 3. (a) Conductivity of various DES-gels at different concentration of MCln. In certain range of concentrations, the conductivity becomes much larger under a higher salt concentration. However, the conductivity shows a downward trend when the concentration of salt increases further. (b) Conductivity of [PG-CaCl2-0.5]-gel at different temperatures. Conductivity increases as the temperature increases](/cms/asset/7b0955d9-6a97-4f0d-8693-f3974523f0d0/tsnm_a_1972053_f0003_c.jpg)
Figure 4. Mechanical properties of various DES-gels with the different concentrations of MCln. (a) [PG-NaCl-x]-gels. (b) [PG-MgCl2-x]-gels. (c) [PG-CaCl2-x]-gels. (d) [PG-AlCl3-x]-gels. The mechanical properties of DES-gels are influenced by the concentration of salt. (e) [PG-CaCl2-1.5]-gel under different temperature. The specimen illustrates higher tensile strength and Young’s modulus under the lower temperature. (f) Cyclic tensile test of the [PG-CaCl2-1.5]-gel under different temperatures. The sample shows an obvious hysteresis under −50°C, indicating a significant energy dissipation mechanism
![Figure 4. Mechanical properties of various DES-gels with the different concentrations of MCln. (a) [PG-NaCl-x]-gels. (b) [PG-MgCl2-x]-gels. (c) [PG-CaCl2-x]-gels. (d) [PG-AlCl3-x]-gels. The mechanical properties of DES-gels are influenced by the concentration of salt. (e) [PG-CaCl2-1.5]-gel under different temperature. The specimen illustrates higher tensile strength and Young’s modulus under the lower temperature. (f) Cyclic tensile test of the [PG-CaCl2-1.5]-gel under different temperatures. The sample shows an obvious hysteresis under −50°C, indicating a significant energy dissipation mechanism](/cms/asset/e2f4e4ad-3671-4eb9-9d77-89034c3f79bb/tsnm_a_1972053_f0004_c.jpg)
Figure 5. Environmental stability of various DES-gels and PAAm-MCln-y hydrogels. (a) Plot of weight maintenance of the DES-gels under 5– 9% relative humidity versus time. The weight of DES-gels can maintain approximately 90% of their initial weights, showing high environmental stability even in an extremely dry condition. (b-e) Images of the DES-gels before and after storage for 7 days. The DES-gels show size stability. (f) Change of weight of the PAAm-MCln-y hydrogels for 24 h. The weight drops persistently fast as water evaporates. (g-j) Photos of the PAAm-MCln-y hydrogels before and after stored for 24 h. The sizes of all PAAm-MCln-y hydrogels are reduced remarkably due to water loss. Relative humidity: 30– 35%, temperature: 20– 25°C. (k) Change of conductivity of DES-gels under 5– 9% relative humidity versus time. Relatively stable conductivity further Proves the high environmental stability of the DES-gels
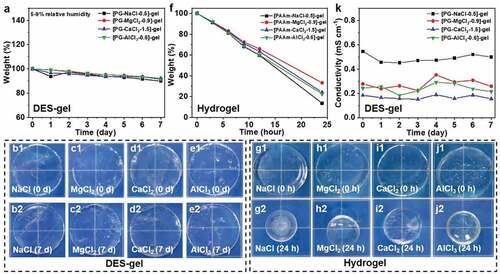
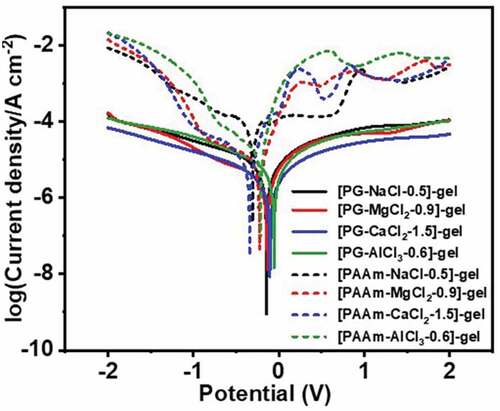
Figure 6. The chemical stability of various DES-gels and PAAm-MCln-y hydrogels. (a-d) Photos of the DES-gels-copper junctions after storing for 7 days. DES-gels are not chemically corrosive to copper sheets, indicating good chemical stability. (e-f) Photographs of PAAm-MCln-y hydrogels-copper junctions after storing for 7 d in a confined space. The hydrogels severely corrode the copper sheets
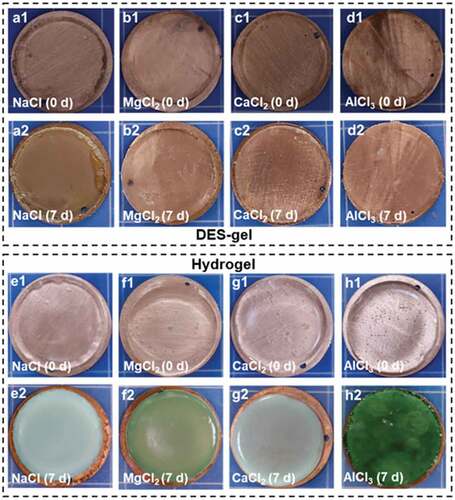
Figure 8. [PG-CaCl2-0.5]-gel applied in stretchable and flexible electroluminescent devices at room and cold environment. (a) Diagram of the as-prepared electroluminescent device. (b) Luminance of the prepared electroluminescent device as a function of temperature, showing that luminance becomes brighter as the temperature increases. (c) Photos of the prepared electroluminescent device in darkroom at 20°C and −20°C. Images of the prepared electroluminescent device in darkroom at 20°C and −20°C with and without (d) stretching and (e) bending. The flexible and stretchable electroluminescent device works in both ambient and cold environments
![Figure 8. [PG-CaCl2-0.5]-gel applied in stretchable and flexible electroluminescent devices at room and cold environment. (a) Diagram of the as-prepared electroluminescent device. (b) Luminance of the prepared electroluminescent device as a function of temperature, showing that luminance becomes brighter as the temperature increases. (c) Photos of the prepared electroluminescent device in darkroom at 20°C and −20°C. Images of the prepared electroluminescent device in darkroom at 20°C and −20°C with and without (d) stretching and (e) bending. The flexible and stretchable electroluminescent device works in both ambient and cold environments](/cms/asset/c6608032-10c3-43ca-b105-cf1ee690ee70/tsnm_a_1972053_f0008_c.jpg)