Figures & data
Figure 1. G-type and V-type agent names and structures. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).
![Figure 1. G-type and V-type agent names and structures. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).](/cms/asset/19af3667-09e9-4341-8bf1-e96c03bcf3cb/tsnm_a_2385350_f0001_b.gif)
Figure 2. Names and structures of the basic mononuclear phosphorus hydrides, hydroxides, and oxides with three and five values. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).
![Figure 2. Names and structures of the basic mononuclear phosphorus hydrides, hydroxides, and oxides with three and five values. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).](/cms/asset/2a62205b-c9f4-4be6-b682-563b756f2df7/tsnm_a_2385350_f0002_c.jpg)
Figure 3. Various structures of OP-based pesticides. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).
![Figure 3. Various structures of OP-based pesticides. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).](/cms/asset/cd390112-738e-47e6-8e90-9c9975af1f68/tsnm_a_2385350_f0003_b.gif)
Figure 4. Names and structures of some pertinent mimics that are utilized in sensing as sarin, VX, and other mimics. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).
![Figure 4. Names and structures of some pertinent mimics that are utilized in sensing as sarin, VX, and other mimics. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).](/cms/asset/19c3065b-86be-48bc-8c3f-5c31a1651cac/tsnm_a_2385350_f0004_b.gif)
Table 1. Advantages and disadvantages of these conventional methods.
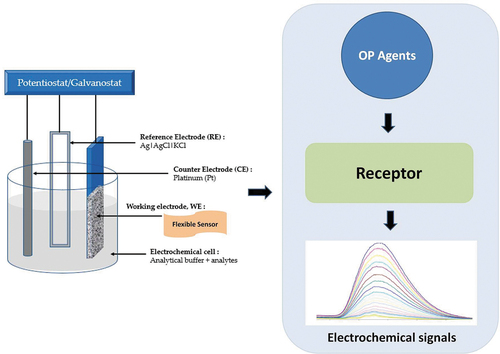
Figure 5. Diagrammatic representation of a traditional electrochemical sensor. Reproduced from [Citation57] under common creative license, MDPI (2020).
![Figure 5. Diagrammatic representation of a traditional electrochemical sensor. Reproduced from [Citation57] under common creative license, MDPI (2020).](/cms/asset/a852ec4f-60ce-44c3-90c1-86e5c3c6cc76/tsnm_a_2385350_f0005_c.jpg)
Figure 6. Diagrammatic depiction of common sensor parts. Reproduced from [Citation71] under common creative license, MDPI (2023).
![Figure 6. Diagrammatic depiction of common sensor parts. Reproduced from [Citation71] under common creative license, MDPI (2023).](/cms/asset/ba98a937-6935-4573-a149-ab29aaf7c125/tsnm_a_2385350_f0006_c.jpg)
Figure 7. (a) electrochemical sensor produced by photolithography and manufacturing process schematic [Citation85,Citation87]. Reproduced with permission from, ref [Citation85], copyright @ Elsevier (2011) and ref [Citation87], copyright @ Wiley (2008). (b) SPE production steps; reproduced from [Citation88] under common creative license, MDPI (2020); (c) an electrochemical sensor made entirely of roll-to-roll graphene nanoplatelets and ZrO2; reproduced with permission from ref [Citation89], copyright @ American Chemical Society (2021).
![Figure 7. (a) electrochemical sensor produced by photolithography and manufacturing process schematic [Citation85,Citation87]. Reproduced with permission from, ref [Citation85], copyright @ Elsevier (2011) and ref [Citation87], copyright @ Wiley (2008). (b) SPE production steps; reproduced from [Citation88] under common creative license, MDPI (2020); (c) an electrochemical sensor made entirely of roll-to-roll graphene nanoplatelets and ZrO2; reproduced with permission from ref [Citation89], copyright @ American Chemical Society (2021).](/cms/asset/91d10f23-4861-44d0-9ad0-995b2c8b2bb7/tsnm_a_2385350_f0007_c.jpg)
Figure 8. Images of the biosensor along with real-time monitoring of methyl parathion. Reproduced with permission from ref [Citation103], copyright @ Elsevier (2020).
![Figure 8. Images of the biosensor along with real-time monitoring of methyl parathion. Reproduced with permission from ref [Citation103], copyright @ Elsevier (2020).](/cms/asset/9e97c3c3-ef05-47cd-9ca2-f3d0900b3f2e/tsnm_a_2385350_f0008_c.jpg)
Figure 9. Image of the wearable glove sensor and real time monitoring of carbendazim, diuron, paraquat, and fenitrothion in cabbage, apple, and orange juice. Reproduced with permission from ref [Citation104], copyright @ Elsevier (2021).
![Figure 9. Image of the wearable glove sensor and real time monitoring of carbendazim, diuron, paraquat, and fenitrothion in cabbage, apple, and orange juice. Reproduced with permission from ref [Citation104], copyright @ Elsevier (2021).](/cms/asset/c9cdea10-9809-4678-97a4-d3f0a0287cf9/tsnm_a_2385350_f0009_c.jpg)
Figure 10. Images of the tattoo-type sensor and its electrochemical performance. Reproduced with permission from ref [Citation105], copyright @ Elsevier (2018).
![Figure 10. Images of the tattoo-type sensor and its electrochemical performance. Reproduced with permission from ref [Citation105], copyright @ Elsevier (2018).](/cms/asset/ed7ba0d0-8226-40c5-a843-554723554d74/tsnm_a_2385350_f0010_c.jpg)
Figure 11. Office paper-based electrochemical sensor with (a) printing method, and (b) device performance towards paraoxon-ethyl [Citation109]. Reproduced with permission from ref [Citation109], copyright © 2021, American Chemical Society. (c) Textile-based sensor for the detection of DFP. Reproduced with permission from ref [Citation110], copyright © 2021 Elsevier B.V.
![Figure 11. Office paper-based electrochemical sensor with (a) printing method, and (b) device performance towards paraoxon-ethyl [Citation109]. Reproduced with permission from ref [Citation109], copyright © 2021, American Chemical Society. (c) Textile-based sensor for the detection of DFP. Reproduced with permission from ref [Citation110], copyright © 2021 Elsevier B.V.](/cms/asset/8104008d-77b7-4e8a-8548-cf8d6ceab432/tsnm_a_2385350_f0011_c.jpg)
Table 2. Electrochemical sensors for organophosphate detection.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.