Figures & data
Figure 1. Dendritic Cells mediate PSA induction of Human CD39+Foxp3+ CD4+ T cells. PSA-mediated induction of CD39+Foxp3+ T cells was observed in the presence of DCs but not other APCs. 3 × 104 NCD4s were cultured in the presence or absence of 25 µg/ml PSA or Sp1 and 100 U/ml IL-2, alongside 5 × 103 of one of 3 primary autologous APC populations: CD19+ B cells, CD14+ monocytes, or total blood dendritic cells. (A) Fold induction of Foxp3+ frequency alone or in the presence of autologous DCs, n = 4. (B) Representative histograms showing frequency of Foxp3+ cells (red line) as an overlay against isotype control (shaded blue), panels were pre-gated on CD4+ cells. (C) Replicates of CD4+Foxp3+ frequency, n = 3. (D) Representative FACS plots showing frequency of CD25 and CD39 cells, pre-gated on Foxp3+ cells. Positive cells (red line) are overlaid against respective isotype controls (shaded blue). (E) PSA dose response of CD4+CD39+Foxp3+ T cells, n = 1 (Bars represent means of 4 culture wells per condition, error bars represent standard error of the mean). (F) Individual repeats of CD39+Foxp3+ T cells, n = 4–14 per condition. P values were calculated using 2-tailed Student's t-test (A, C) or one-way ANOVA using Dunnett's multiple comparison test (E, F).
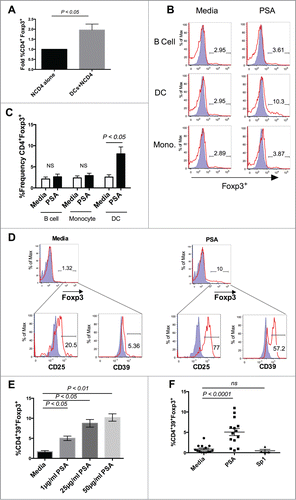
Figure 2. PSA induction of CD39+Foxp3+ T cells requires engagement of HLA-DR and costimulatory molecules. Blocking HLA-DR or costimulatory molecules limited PSA-mediated induction of CD39+Foxp3+ T cells and cytokine production. (A) 5 × 104 DCs were cultured with 25 µg/ml PSA for approximately 16 hours before being stained for surface expression of the above markers, n = 3–6. DCs were incubated with 10 µg/ml of blocking antibody specific to CD86, PD-L1, HLA-DR or CD40L for 30 min prior to co-culture with 3 × 104 autologous NCD4s in the presence or absence of 25 µg/ml PSA and 100 U/ml IL-2. Supernatants were assessed by ELISA for IL-10 or IFNγ. (B) CD4+CD39+Foxp3+ T cell frequency, n = 6–10 per condition (C) IL-10 and IFNγ production, n = 4–10 per condition. Error bars reflect standard error of the mean representing independent experiments, each using cells from different individuals. P values were calculated using 2-tailed Student's t-test (A) or one-way ANOVA using Dunnett's multiple comparison test (B, C).
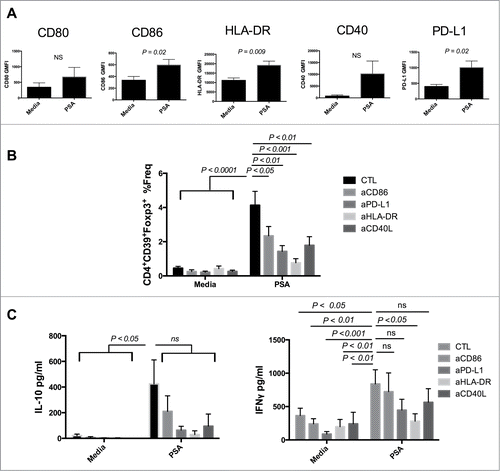
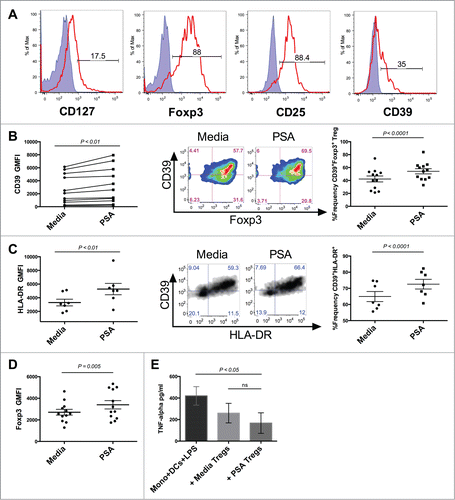
Figure 3. PSA increases the frequency of CD39-expressing Tregs and Treg function. PSA exposure significantly enhanced the frequency of CD39 and HLA-DR-expressing circulating Tregs and resulted in greater capacity to suppress monocyte secretion of TNFα. 1 × 104 Tregs were cultured in the presence or absence of PSA and 100 U/ml IL-2, alongside 5 × 103 autologous DCs. (A) Representative histograms of different Treg-associated markers. Positive cells (red line) are overlaid against respective isotype controls (shaded blue) (B) CD39 MFI, n = 11 (left), representative CD39+Foxp3+FACS panel pre-gated on CD4+ cells (center), replicates of CD39+Foxp3+Treg frequency, n = 12 (right). (C) HLA-DR MFI, n = 7 (left), Representative CD39+HLA-DR+ Treg FACS panel pre-gated on CD4+Foxp3+ cells (center), replicates of CD39+ HLA-DR+ Treg frequency, n = 7 (right). (D) Foxp3 MFI of CD4+ Tregs, n = 12 (E) Suppression of LPS-induced TNFα production, n = 3. Error bars reflect standard error of the mean representing independent experiments, each using cells from different individuals. P values were calculated using 2-tailed Student's t-test (B–D) or one-way ANOVA using Dunnett's multiple comparison test (E).