Figures & data
Figure 1. DOXO induces apoptosis in intestinal epithelium irrespective of the presence of enteric bacteria. A. H&E images demonstrating mitotic bodies and immunofluorescence staining indicating the presence of active caspase 3-positive cells (green) 6h following DOXO treatment in both CONV and GF mice. Arrows indicated apoptotic cells. B. Quantitation of the number of apoptotic cells per crypt, for a total of 20 crypts per animal, in CONV and GF jejunal tissue from control mice and 6, 72, and 120 h after DOXO treatment. * indicates values significantly different from their respective 0h controls p ≤ 0 .05. C. Positional analysis of apoptotic bodies in jejunal epithelium from CONV and GF mice 6h following DOXO treatment. Scale bar: 30 μm.
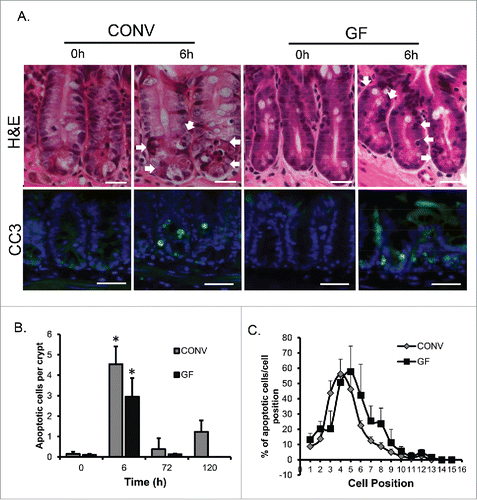
Figure 2. DOXO treatment does not alter crypt depth or villus height in GF mice. A. Micrographs of representative H&E stained sections from GF and CONV mice of control tissue and 6, 72 and 120 h following DOXO treatment. B. Quantitation of crypt depth on 10 15 crypts/villi in CONV and GF jejunal tissue from control mice and 6, 72, and 120 h after DOXO treatment. * indicates values significantly different from their respective controls p ≤ 0 .05. # indicates values significantly different within a particular time point p ≤ 0 .05. C. Quantitation of villus height in CONV and GF jejunal tissue from control mice and 6, 72, and 120 h after DOXO treatment. Scale bar: 50 μm.
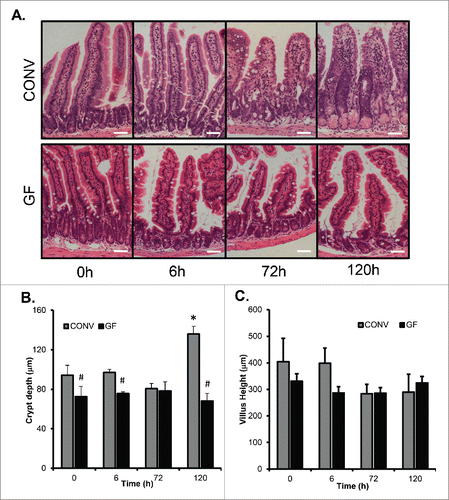
Figure 3. DOXO treatment does not impact proliferation or mitotic index in jejunal epithelium of GF mice. A. Micrograph showing representative staining for pHH3 on jejunal sections from GF and CONV mice. B. Quantitation of pHH3+ cells in CONV and GF jejunal tissue from control mice and 6, 72, and 120 h after DOXO treatment. * indicates values significantly different from their respective controls p ≤ 0 .05. # indicates values significantly different within a particular time point p ≤ 0 .05. C. Quantitation of mitotic index in CONV and GF jejunal tissue from control mice and 6, 72, and 120 h after DOXO treatment. # indicates values significantly different within a particular time point p ≤ 0 .05. ND means “not detected.” Scale bar: 50 μm.
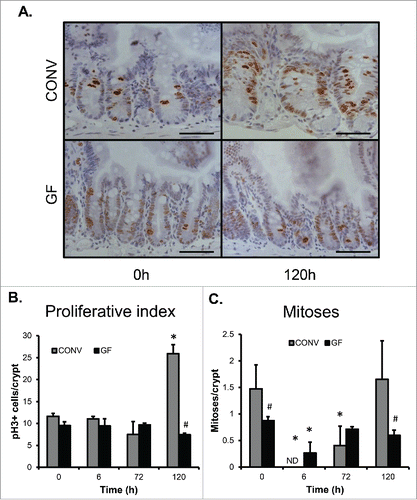
Figure 4. DOXO treatment does not alter crypt density in jejunal mucosa of GF mice. * indicates values significantly different from their respective controls p ≤ 0 .05. # indicates values significantly different within a particular time point p ≤ 0 .05.
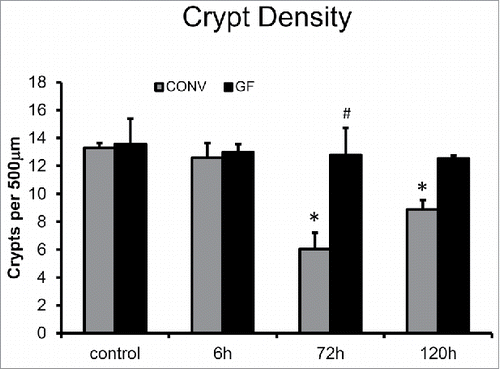
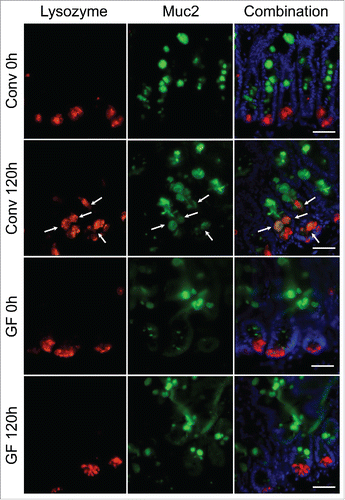
Figure 5. Expansion of the Paneth cell compartment and allocation of intermediate cells are not observed in GF mice following DOXO. Immunofluorescent detection of lysozyme (red), muc2 (green), and nuclei (blue) in jejunal sections from GF and CONV mice. Arrows indicate ‘intermediate cells’, characterized by their co-expression of lysozyme (red) and muc2 (green). Scale bar: 50 μm.