Figures & data
Figure 1. Contributions of gut commensal bacteria and their metabolites to the host immune system. Based on the function of antigen-presenting cells, such as DCs, NK cells, and macrophages, commensal bacteria mediate the differentiation of naïve CD4 + T cells into different subgroups, such as T-bet+ Th1 cells, GATA3+ Th2 cells, RORγt+ Th17 cells, and FOXP3+ Tregs, which further contribute to different immune modulation responses, and the production of various cytokines, such as TGF-β, IFN-γ and ILs. Immune regulation can be mediated not only by bacteria but also by their metabolites, especially SCFAs and AHR ligands, exerting functions by binding GPCRs and AHR on the surface of epithelial cells and immune cells, respectively, which subsequently contribute to augmented epithelial barrier function and improved gut immune tolerance. Conversely, some immune cells and epithelial cells can also mediate the balance of bacteria by secreting antibacterial substances, such as B cells secreting IgA, Goblet cells secreting mucins and Paneth cells secreting antimicrobial peptides, etc. Overall, microbiota-immune cross-talk contributes to gut homeostasis by forming a relatively stable feedback loop. SCFAs, short-chain fatty acids; AHR, aromatic hydrocarbon receptor; GPCRs, G protein-coupled receptors; TCR, T cell receptor; PRR, pattern recognition receptor; DAMP, damage-associated molecular pattern; MHC-II, major histocompatibility complex II; B7, B7.1(CD80)/B7.2(CD86); DC, dendritic cell; NK, natural killer cell; NE, neutrophil; Treg, regulatory T cell; CTL, cytotoxic T lymphocyte; ILC, innate lymphoid cell; IFN-γ, interferon-γ; TGF-β, transforming growth factor; and IL, interleukin.
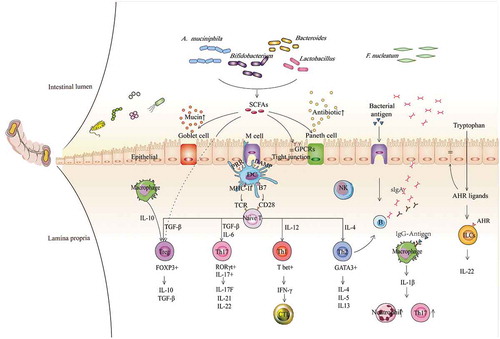
Figure 2. Potential mechanism that explains the anticancer or pro-cancer effects of some candidate ICI-therapy-associated bacteria by shaping the host immune status. Lactobacilli may upregulate the expression of MHC-II on DCs, enhance the activity of NK cells and macrophages, and improve Th1-mediated immune responses as well as increase the production of IFN-γ in tumors. The effects described above facilitate the anticancer potential of Lactobacilli. Bifidobacteria can improve the efficacy of anti-PD-L1 therapy by upregulating the expression of MHC-II on DCs, promoting Th1 polarization and CTL accumulation in the tumor microenvironments, and reducing the toxicity of anti-CTLA-4 therapy through enhanced Treg cell metabolism. Akkermansia muciniphila can enhance the efficacy of anti-PD-1 therapy in a manner dependent on the enhanced IL-12-dependent Th1-related immune response, along with increased levels of IFN-γ and TNF-α and decreased levels of IL-4 and IL-10. The results from a preclinical trial confirmed that Bacteroidetes can restore anti-CTLA-4 treatment efficacy by enhancing the IL-12-dependent Th1-related immune response, whereas these bacteria are associated with poor clinical outcomes of anti-CTLA-4 or anti-PD-1 therapy in human clinical trials. Fusobacterium nucleatum can promote tumor progression in gastrointestinal cancer and pancreatic cancer, and it is verified to be associated with reduced density of CD3 + T cells, exhaustion of NK cells, and augmentation of M2 polarization along with accumulation of MDSCs in tumor microenvironments. These effects may be closely linked with their colonization in tumors.
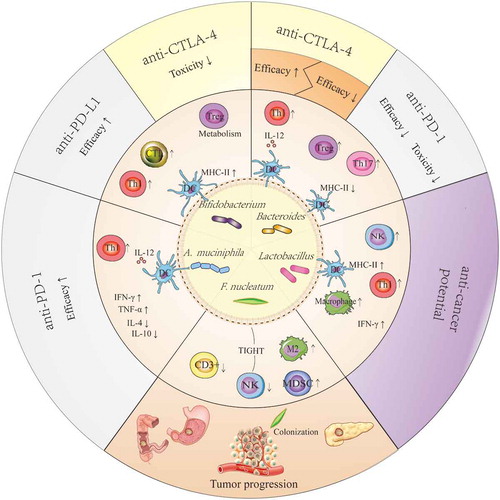
Table 1. Effects of potential immune-associated bacteria on the host immune system.
