Figures & data
Figure 1. Normal maturation process of early gut microbiota in vaginally delivered, full-term, and breast-fed infants. For bacterial microbiota, the abundance of Proteobacteria is highest during the first few weeks of life, but decreases quickly over time. Actinobacteria, primarily certain Bifidobacterium strains, initially increase followed by a decrease, reaching its highest level of richness at around six months of age. The abundance of Bacteroidetes is low at birth but increases steadily during the first year of life. The overall proportion of Firmicutes does not change significantly, showing some fluctuations during maturation. Verrucomicrobia, mainly Akkermansia muciniphila, can be detected after one month of age, and its abundance increases over time. For viral microbiota, virus-like particles cannot be detected at birth but their richness increases quickly and reaches 109/g feces at four months of age, a level similar to that in adults. Furthermore, an increase in virus infecting human cells also becomes evident over time. For mycobiota, its α-diversity shows a slight increase over time, while its β-diversity remains mostly unchanged. However, the mycobiota composition changes over time, showing a shift from the early dominance of Debaryomyces hanseni to Saccharomyces cerevisiae at one year of age. Moreover, changing GM patterns can vary substantially among individuals and can be disrupted by various factors. Therefore, a precise time frame regarding the maturation of early GM compositions needs to be established
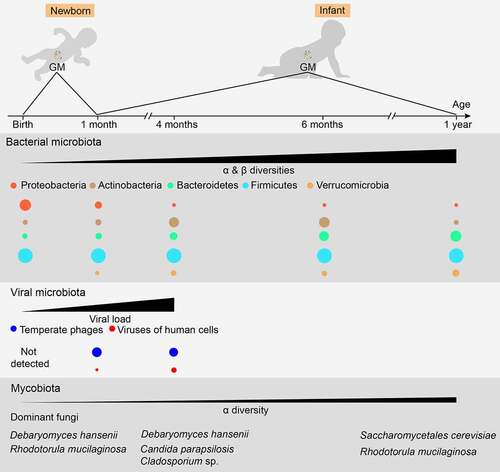
Figure 2. The role of milk SIgA in regulating gut microbiota maturation. IgA+ plasma cells (PCs) produce milk SIgA in the mammary gland, which originates from the gut and is educated by gut microbiota (GM). Milk SIgA is the primary (exclusive) source of intestinal SIgA for breastfeeding infants. SIgA in the intestine can bind to specific pathogens, promoting their clearance via aggregation. The binding of SIgA to certain commensals, such as Lactobacillus, can enhance their mucosal colonization. A lack in milk SIgA can lead to the over-enrichment of pathogens and delayed GM maturation, which is associated with the development of various microbe- and immunity-related diseases during infancy (for example, NEC and IBD) and in later life (for example, obesity, allergies, and autoimmune diseases). NEC, necrotizing enterocolitis; IBD, inflammatory bowel disease
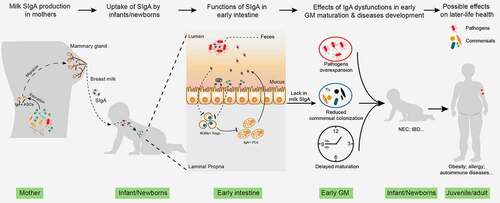
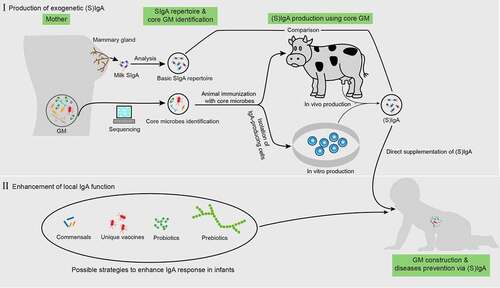
Figure 3. Possible application of IgA to enhance normal gut microbiota maturation. before direct iga supplementation, the “core microbes” must first be identified by analyzing the gut microbiota (GM) properties in pregnant women based on large-scale sequencing. This core microbiota is then used to immunize cows or IgA-producing antibody-secreting cells (ASCs) (or through other possible methods) and obtain IgA. Next, a comparison between the repertoires of this IgA and milk SIgA is also required. For enhancing local IgA functionality, the possible approaches include the supplementation of specific commensals, probiotics, prebiotics, and unique vaccines derived from pathogens