Figures & data
Table 1. Histological score of colon mice
Figure 1. Transient C. rodentium infection induces persistent perturbations in mice.
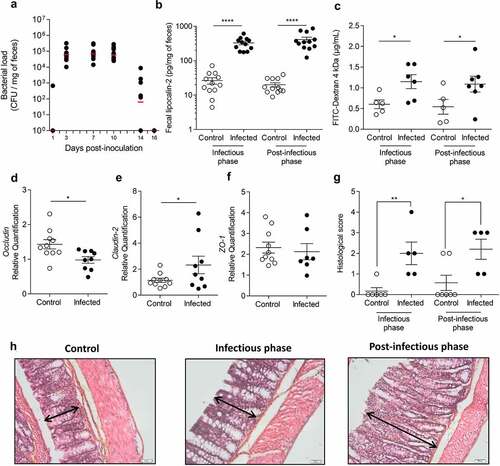
Figure 2. Post-infectious visceral sensitivity and anxiety-like behavior induced by C. rodentium.
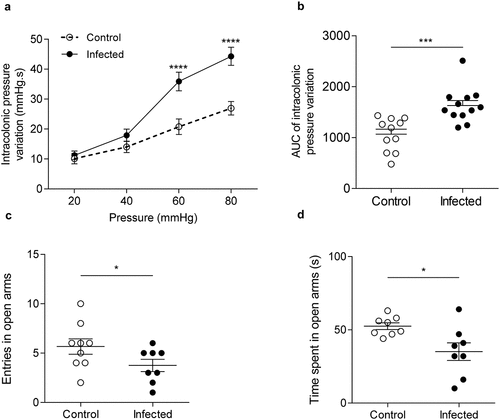
Figure 3. Colonic mucosa-associated microbiota dysbiosis after C. rodentium clearance in mice.
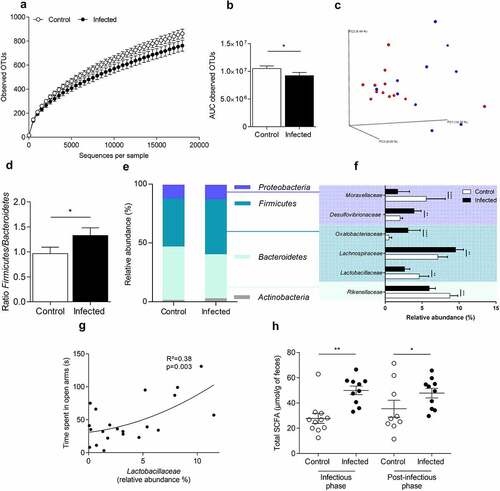
Figure 4. AhR activity and tryptophan metabolic pathways are modified in post-infectious period.
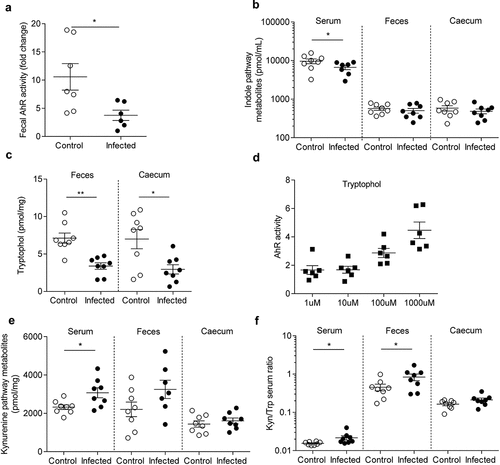
Figure 5. IL-22 treatment reverses post-infectious anxiety-, cognition-like behaviors and ill-being induced by C. rodentium infection.
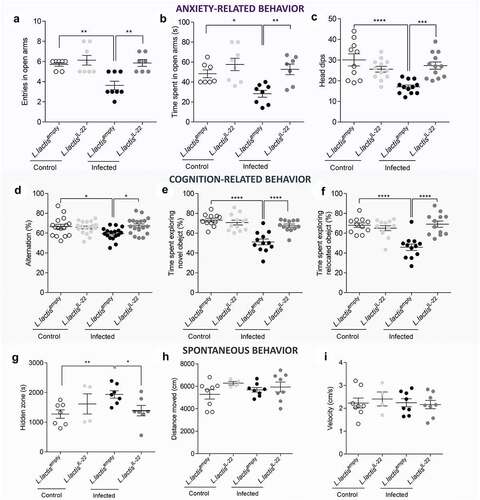
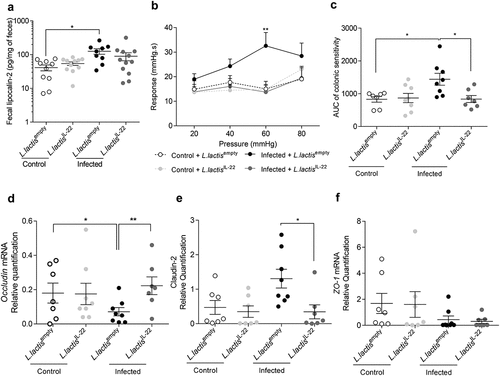
Figure 6. IL-22 treatment reverses post-infectious CHS and intestinal barrier alteration induced by C. rodentium infection independently of inflammation.