Figures & data
Figure 1. Effects of bacteriophage inoculation on the intestinal flora. ① Phages T4, F1, B40-8, and VD13 lysed only their susceptible bacteria E. coli, C. sporogenes, B. fragilis and E. faecalis. Phages directly affect susceptible bacteria through ① specific lysis, ② horizontal gene transfer and ③ encoding virulence genes, resulting in changes in intestinal bacteria and a decrease in their number. ④ The type of metabolites secreted may change due to the change in bacterial characteristics, and the total amount of metabolites secreted may decrease due to the decrease in bacterial number. ⑤ Changes in the abundance of bacteria and their metabolites can affect the intestinal environment and the growth of surrounding bacteria.
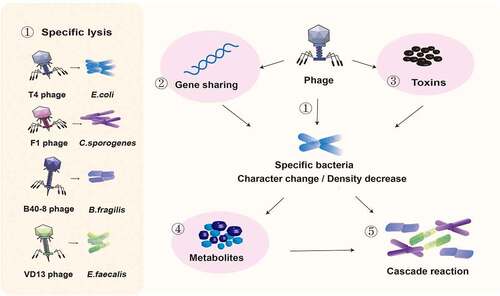
Figure 2. Antagonistic mechanisms of intestinal bacteria against phage invasion. ① Bacteria can prevent phage adsorption by changing and hiding receptors. ② Bacteria destroy the DNA of the invading phage through the restrictive modification-methylation pathway and inhibit the replication and integration of phage genes. ③ Bacteria degrade phage DNA by the CRISPR-Cas system. ④ Bacteria prevent reinfection by encoding superinfection immune system proteins. ⑤ Bacteria interfere with phage adsorption, injection, replication, assembly and release through the Abi system, leading to failure of phage lysis.
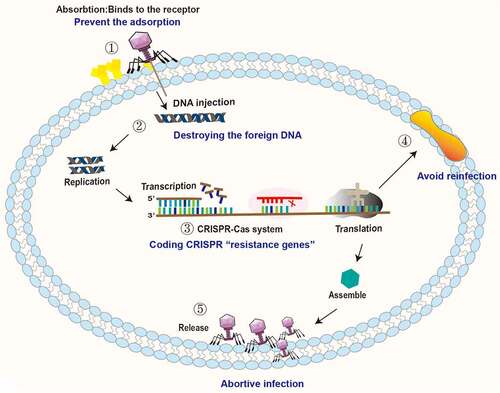
Figure 3. The cause of the persistent coexistence of phages and bacteria in the intestinal tract. ① The distribution of bacteriophages and bacteria is spatially heterogeneous. There are no phages in the intestinal villi, few phages in the intestinal mucosa, and a large number of phages in the intestinal lumen. The phage density in the intestinal tract presents a mucosal-lumenal gradient, providing a place for bacteria to avoid phage attack. ② The specific interaction between phages and bacteria, the competition between defense and anti-defense systems, and the way in which genes are shared to form new mutations or new species promote coevolution.
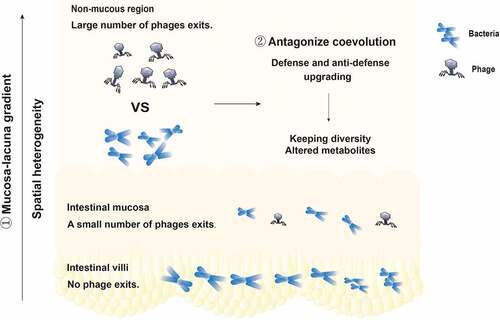
Figure 4. Intestinal phages induce the development of diseases by regulating and increasing the release of bacterial exotoxins, regulating bacterial community metabolism, and initiating immune responses. Changes in intestinal phages, bacteria and their metabolites through fecal microbiota transfer may help alleviate diseases. Changes in bacterial metabolites closely related to disease may be able to be used as markers for disease surveillance.
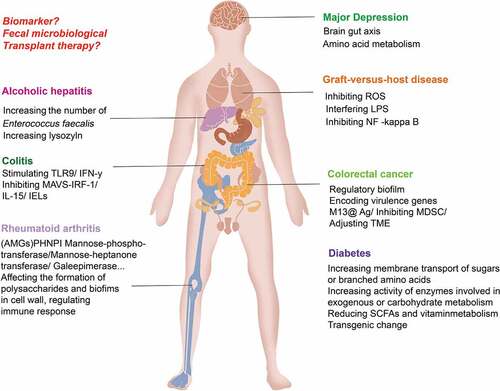
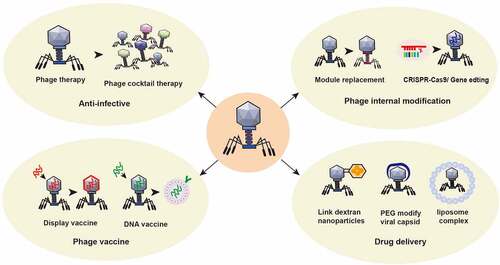
Figure 5. Application of phage in the treatment of intestinal diseases. ① Using the nature of phage, a phage cocktail is made to target bacterial infection. ② The structure of phages can be modified by switching phage modules or by applying CRISPR gene editing technology to change the intestinal bacterial host or enhance its antagonism to the intestinal bacterial host. ③ Preparation of phage display vaccines and phage DNA vaccines to enhance specific immune responses. ④Using phage to treat specific intestinal or other bacteria with targeted delivery of disease treatment drugs. For example, glucan nanoparticles are covalently linked to azide-modified phages, polyethylene glycol capsid-modified phages, phage-liposome complexes and other anti-colorectal cancer drugs.