Figures & data
Figure 1. Schematic representation of secreted and transmembrane mucins. Transmembrane mucins are large and attached to IEC surface via transmembrane and cytoplasmic regions. Cysteine molecules widely present in PTS domains of secreted mucins form intra-disulfide bonds (hydrophobic interactions). In addition, the gel-like structure of secreted mucins is provided by electrostatic interactions within highly glycosylated regions.
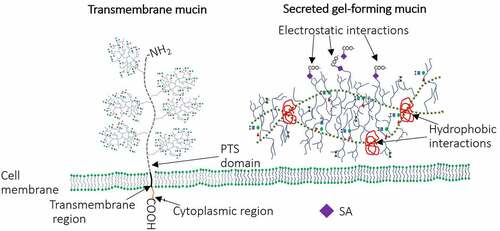
Figure 2. Schematic representation of the mucin family and their structure. Tandem repeat domains – enriched in proline (Pro), threonine (Thr) and/or serine (Ser) (PTS domain) are highly glycosylated [including N-acetylgalactosamine (GalNac), N-acetylglucosamine, fucose, galactose and sialic acid (SA)]. These O-linked glycan domains, represent 50% (w/w) of all mucins.Citation41 Mucin glycosylation occurs within the endoplasmic reticulum and the Golgi apparatus; once glycosylated they are secreted on the apical surface of goblet cells.Citation42 The initial step of mucin-type O-glycosylation in mammals (addition of GalNAc to PTS domain – Tn antigen) is provided by the activity of N-acetylgalactosaminyltransferases (ppGalnac-Ts) - enzymes which are encoded by one of 20 genes. This antigen is further extended by addition of GalNAc, galactose, N-acetylglucosamine and SA (provided by activity of different glycosyltransferases) leading to formation of four different glycan core types that can be included in the structure of MUC1, MUC2, MUC5AC, MUC5B, MUC6–8, MUC 11–13 and MUC 16.Citation39 Activity of FUT2 (active only in secretor individuals) regulates the production of H-type 1 antigen while FUT1 enzyme is responsible for production of H-type 2 antigen (cell-associated antigen). The peripheral terminal region may be presented by l-fucose (Fuc), d-galactose (Gal), N-acetylgalactosamine (GalNac), N-acetylglucosamine (GlcNac) and SA residues, included in the structure of all HBGAs such as A, B, H, Lewis a (Lea), Lewis b (Leb), Lewis x (Lex) and Lewis y (Ley).Citation43.
![Figure 2. Schematic representation of the mucin family and their structure. Tandem repeat domains – enriched in proline (Pro), threonine (Thr) and/or serine (Ser) (PTS domain) are highly glycosylated [including N-acetylgalactosamine (GalNac), N-acetylglucosamine, fucose, galactose and sialic acid (SA)]. These O-linked glycan domains, represent 50% (w/w) of all mucins.Citation41 Mucin glycosylation occurs within the endoplasmic reticulum and the Golgi apparatus; once glycosylated they are secreted on the apical surface of goblet cells.Citation42 The initial step of mucin-type O-glycosylation in mammals (addition of GalNAc to PTS domain – Tn antigen) is provided by the activity of N-acetylgalactosaminyltransferases (ppGalnac-Ts) - enzymes which are encoded by one of 20 genes. This antigen is further extended by addition of GalNAc, galactose, N-acetylglucosamine and SA (provided by activity of different glycosyltransferases) leading to formation of four different glycan core types that can be included in the structure of MUC1, MUC2, MUC5AC, MUC5B, MUC6–8, MUC 11–13 and MUC 16.Citation39 Activity of FUT2 (active only in secretor individuals) regulates the production of H-type 1 antigen while FUT1 enzyme is responsible for production of H-type 2 antigen (cell-associated antigen). The peripheral terminal region may be presented by l-fucose (Fuc), d-galactose (Gal), N-acetylgalactosamine (GalNac), N-acetylglucosamine (GlcNac) and SA residues, included in the structure of all HBGAs such as A, B, H, Lewis a (Lea), Lewis b (Leb), Lewis x (Lex) and Lewis y (Ley).Citation43.](/cms/asset/e6ed8e76-d638-491f-9155-deacdcaa13c1/kgmi_a_2197833_f0002_oc.jpg)
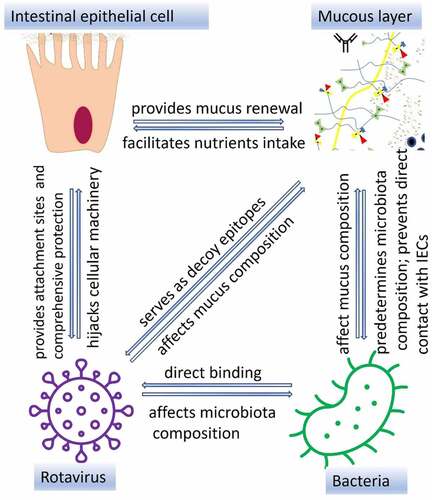
Figure 3. Interactions between IECs and other components of the intestinal mucosa. Interactions between bacterial ligands and Toll-like receptors (TLRs) induce signaling cascades resulting in activation of transcription factor nuclear factor-kappa-chain-enhancer (NF-κb) which lead to increased expression of RegIII proteins, secreted and transmembrane mucins leading to thickening of the mucus layer. Direct contact between RV VP8* and tumor necrosis factor (TNF) receptor associated factor 2 (TRAF2) increases MUC2 transcription. Produced by goblet cells, secreted mucins form two layers: first, dense firmly attached inner layer directly covering IECs surface, non-penetrable for bacteria. Second, loosely attached layer is a habitat for bacteria. Mucin O-glycans within inner and outer layer directly interact with RV and IgA binds RV preventing them to reach IECs. Antibacterial barrier function of mucus layers is further supported by antimicrobial RegIII protein. IgA produced by plasma cells in Peyer’s patches cells block receptors on IEC surface and/or directly bind pathogenic bacteria, immune exclusion, and RV or may facilitate the formation of biofilm (immune inclusion).
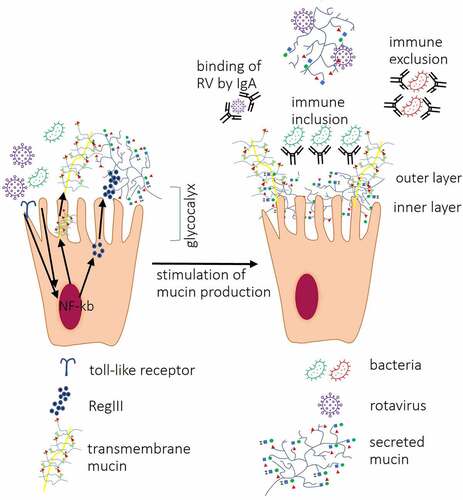
Table 1. A summary of glycan cores, SA-containing glycans and HBGA types recognized by different RVA and RVC genotypes in different hosts.
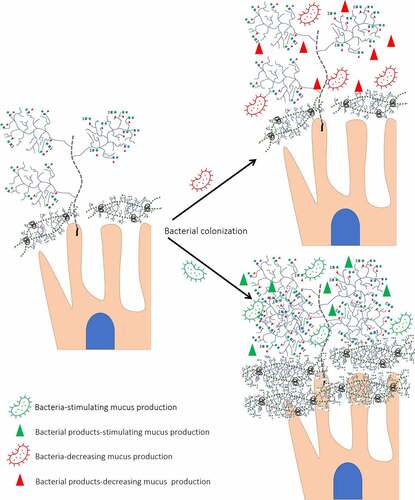
Figure 4. Different effects of bacteria on mucin expression/glycosylation. Presence of glycosyl hydrolases allows bacteria to degrade O-glycans decreasing its protective role against RV infection. In addition, bacterial proteases cleave transmembrane mucins. In contrast, bacteria-stimulating bacteria by interaction with transmembrane proteins increases mucin concentration and glycosylation.