Figures & data
Figure 1. The iron cycle and metabolism. Iron is obtained from the diet in the form of heme or nonheme iron and is absorbed by enterocytes while Fe3+ is reduced to Fe2+ by reductase before being absorbed by the receptor DMT1. Macrophages phagocytose senescent blood cells, degrade heme via heme oxygenase and release free iron. The absorbed iron is then either stored in ferritin, used for the biosynthesis of iron-sulfur clusters, or exported by ferroportin1 with a multi-copper ferroxidase such as ceruloplasmin, which oxidizes Fe2+ to Fe3+ in the plasma membrane. Iron can also be exported with ferritin by multivesicular bodies. Iron in the blood is bound to transferrin, forming an iron-transferrin complex. This complex is transported to the liver for storage, to bone marrow cells for heme synthesis, and to other tissues for the synthesis of iron-containing enzymes. Cells take up iron through transferrin receptor 1-mediated endocytosis. Fe3+ is liberated from transferrin and subsequently reduced to Fe2+ by endosomal reductases. Hepcidin, secreted by the liver, either inhibits the degradation of ferroportin1 or directly blocks the channel, allowing iron to accumulate within the cell. DMT1, divalent metal-ion transporter-1.
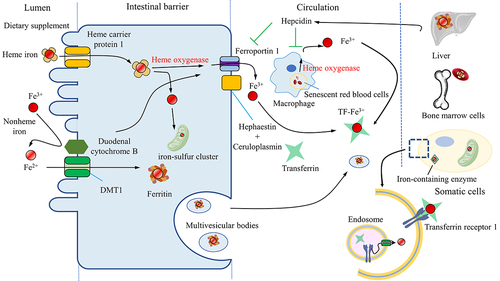
Table 1. The compositions or metabolites of microbiota affect ferroptosis.
Figure 2. Intestinal microbiota affects iron metabolism. Microorganisms utilize various mechanisms to obtain different forms of iron (Fe3+, Fe2+, heme iron). Moreover, the microbiota reduces intestinal pH by producing essential amino acids or SCFAs to optimize dietary iron bioavailability, regulates the expression of hepcidin, transforms inorganic iron into organic forms to lower the toxicity of free iron and modulates the ferritin levels. The microbiota also activates the HO-1/CO pathway or NRF2/HO-1 to strengthen phagocytosis and increase macrophage processing of hemoglobin for iron release. SCFAs, Short-chain fatty acids; HO-1, heme oxygenase-1; CO, carbon monoxide; NRF2, Nuclear factor erythroid 2-related factor 2.
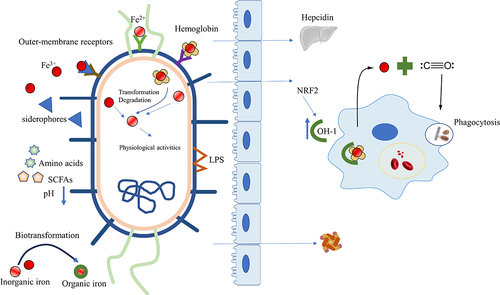
Figure 3. Intestinal microbiota affects oxidative stress. The induction of oxidative stress can be attributed to the iron-dependent Fenton reaction, as well as enzymes belonging to the NOX family and mitochondria. Microbes can remove substrates of the Fenton reaction, assimilate Fe2+, and decompose H2O2 using enzymes synthesized by the bacteria themselves. Microbes can also enhance mitochondrial functions, including oxidative phosphorylation and β-oxidation, under physiological stress or mitochondrial dysfunction by activating the PGC1α signaling pathway. Microbes can increase NOX1 levels and activate the NRF2 pathway, while suppressing histone deacetylase activity to prevent ROS formation. Additionally, SOD in microbes can reduce ROS levels in the host. The level of ROS can also affect the microbes themselves. NOX, nicotinamide adenine dinucleotide phosphate oxidase; H2O2, Hydrogen peroxide; PGC1α, peroxisome proliferator-activated receptor-gamma coactivator-1alpha; NRF2, Nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen stress; SOD, superoxide dismutases.
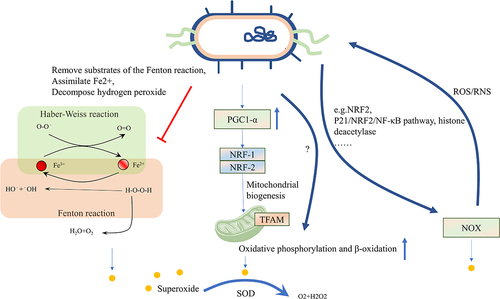
Figure 4. Fatty acid metabolism and intestinal microbiota. Exogenous PUFAs increase sensitivity to ferroptosis and shape the gut microbiota. The gut microbiota influences the balance of PUFAs by regulating lipid synthesis, degradation, and biotransformation. Microbiota can influence the expression of ACSL4, disturbing the esterification of PUFAs or facilitating the transformation of saturated fatty acids or PUFAs-derived intermediate metabolites, thereby influencing sensitivity to oxidation. PUFAs, Short-chain fatty acids; ACSL4, acyl-CoA synthetase long-chain family 4.
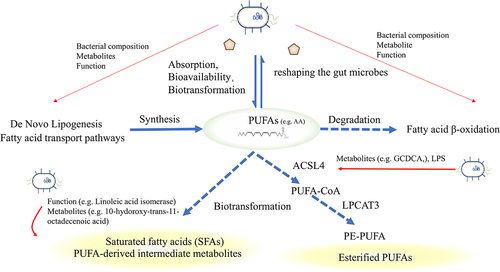
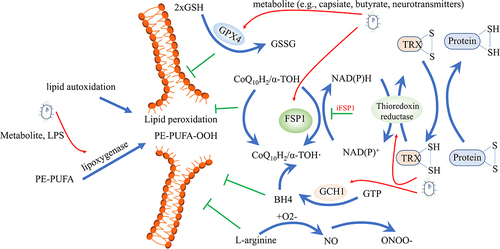
Figure 5. Intestinal microbiota affects lipid peroxidation. Different antioxidant systems are effective in preventing lipid peroxidation, including the cysteine/GSH/GPX4 axis, the FSP1-CoQ10 axis, the GCH1-BH4 axis, the thioredoxin system, and the iNOS/NO• system. On the other hand, lipid autoxidation and lipoxygenase can pose dangerous risk factors for inducing lipid peroxidation. Gut microbes can regulate both the antioxidant systems and lipoxygenase, influencing ferroptosis. GSH, glutathione; GPX4, glutathione peroxidase 4; FSP1, ferroptosis suppressor protein 1; CoQ10, Ubiquinone; GCH1, GTP cyclohydrolase-1; BH4, Tetrahydrobiopterin; iNOS, inducible nitric oxide synthase.