Figures & data
Figure 1. Relationships between gut microbiota metabolites and macrophage polarization. (a) intestinal flora utilizes LPS as a trigger to regulate the aggregation of monocyte-like macrophages through the LPS/TLR4 pathway. This process leads to the formation of a precancerous inflammatory microenvironment that promotes the polarization of M1 macrophages. The process of aging results in the growth of the Firmicutes or Aspergillus phylum in the intestinal microbiota. This, in turn, leads to an increase in LPS and continuous stimulation of inflammatory signalling pathways in host intestinal macrophages. As a result, pro-inflammatory cytokines TNF, IL-1β and IL-6 are upregulated, NF-κB activation is induced, and M1-like polarization is facilitated. Microglia exhibits an M2-like polarized phenotype as the secretion of pro-inflammatory mediators such as TNF-α, NO, PGE2, IL-1 and 6 is reduced after LPS pretreatment and exposure to LPS stimulation. Additionally, M2 markers such as ARG1 and IL-10 are upregulated. CARKL inhibits the LPS-induced expression of SOCS3, indirectly increasing STAT3 phosphorylation. It also upregulates the expression of immune checkpoint molecules PD-L1 and CTLA-4, and facilitates polarization of M2-like macrophages. (b) low concentrations of SCFAs have been found to suppress pro-inflammatory factors such as TNF-α, IL-1/6 and iNOS, while increasing anti-inflammatory factors like IL-10. This leads to a switch in macrophages towards an M2-like polarization. BHB enhances the expression of M2-related genes, including IL-4/10, ARG1, and CHil3, by strengthening the JAK2-STAT6 signaling pathway, thus directly promotes M2 macrophage polarization. The consumption of complex probiotics has been shown to increase the growth of bacteria that produce SCFAs, specifically butyric and propionic acid. This process also activates the GPR41/43 pathway and the NF-κB cascade response, while inhibiting MyD88 through the TLRs/MyD88/NF-κB signalling pathway. Hence, complex probiotics foster macrophage M2 polarization. Butyrate treatment of macrophages promotes M2 polarization by inhibiting HDAC1 gene expression and enhancing H3K9 acetylation, leading to STAT6 phosphorylation. During recovery from ulcerative colitis, levels of butyrate are greatly increased, which mediates macrophage activation of the WNT-ERK1/2 axis and improves expression of CD206, IL-4, and IL-13, ultimately inducing M2 polarization. (c) DCA induces ecological dysregulation, upregulates MCP-1 expression, and elevates mRNA levels of M2 genes such as ARG-1 and MR, leading to the M2 phenotypic polarization of TAMs. Furthermore, dysbiosis of intestinal flora stimulates IL-25 secretion, which induces polarization of M2 type macrophages. Additionally, DCA increases the mRNA expression level of M2-mAchR in macrophages and promotes M1 macrophage polarization through the TLR2-NF-κB/ERK/JNK pathway. (d) the activation of AhR by IAA is responsible for the M2 TAMs phenotype. SG leads to changes in the intestinal microflora, resulting in an increase in the levels of IAA, which in turn stimulates the polarization of macrophages to the M2 type. FICZ facilitates the expression of AhR, which indirectly leads to an increase in the expression of M2 markers such as ARG-1. This process enhances M2 polarization by downregulating IRF1 and HIF-1α, and inhibiting iNOS expression through the miR-142a-IRF1/HIF-1α pathway. (e) TMAO is created by gut microbes during the breakdown of choline from food. It has been observed to stimulate the production of pro-inflammatory mediators such as TNFα and ROS, which can result in a shift toward the M1 TAM phenotype.
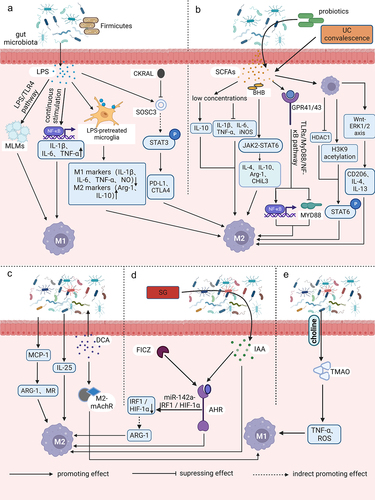
Figure 2. The polarization of TAMs is influenced by gut microbiota through other cells. The metabolites butyric acid and propionic acid produced by intestinal microbes increase the presence of Tregs that express the Foxp3 protein. Tregs indirectly promote the accumulation of M2-like TAMs in the TME by suppressing the secretion of IFN-γ in CD8+ T cells. The imbalance of gut microbiota leads to the release of LPS in large quantities. This, in combination with the overexpression of LPS in the TME, results in a significant increase in the secretion of CTSK. CTSK then binds to TLR4 through an mTOR-dependent pathway, which stimulates TAMs to polarize toward the M2 phenotype.
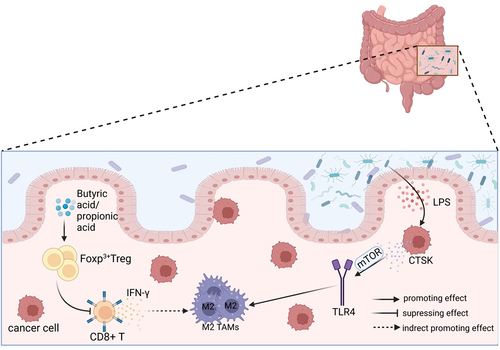
Figure 3. Gut microflora homeostasis and its dysbiosis impact on TAMs. The gut microbiota plays a crucial role in maintaining a balanced immune system and protecting the intestinal epithelium. Under normal conditions, the Firmicutes and the Bacteroides should be present in equal proportions. This balance helps reduce inflammation, cancer incidence, and supports normal body immunity. However, dysbiosis of the intestinal flora disrupts this balance, leading to the excessive activation of TAMs and the secretion of IL-6 and TNF-α. These factors contribute to tumor invasion, metastasis, and accelerated growth through the promotion of EMT. Dysbiosis also results in increased secretion of CTSK and IL-25, which further promote the polarization of M2 type macrophages and tumor development. The imbalance of flora often leads to an increase in harmful bacteria, such as F.Nucleatum. This bacterium can enhance the expression of CCL20, which in turn facilitates the recruitment of TAMs to the TME and promotes tumor growth. Dysbiosis is primarily characterized by a decrease in the proportion of Firmicutes and an increase in the phylum of Bacteroides. These changes can contribute to the polarization of TAMs into M2 type and expedite tumor growth.
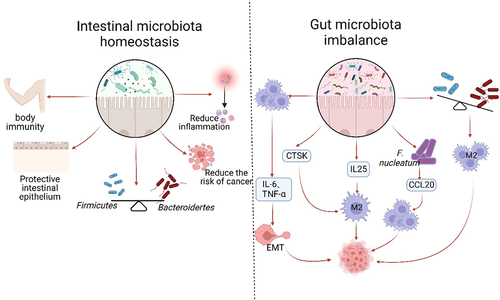
Table 1. Presentation and markers of gut microbiota modulating on TAMs in different tumors.
Table 2. Partial information on clinical trials on gut microbiota and cancer immunotherapy.
Data availability statement
All data are included in the manuscript.
