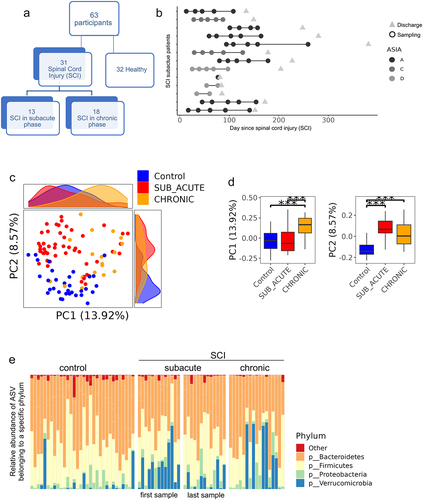
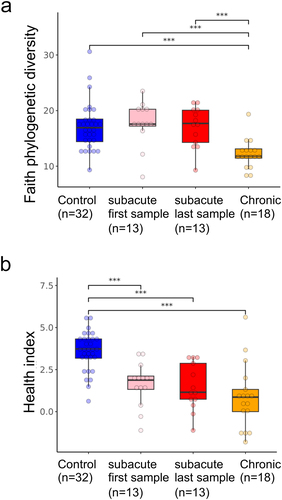
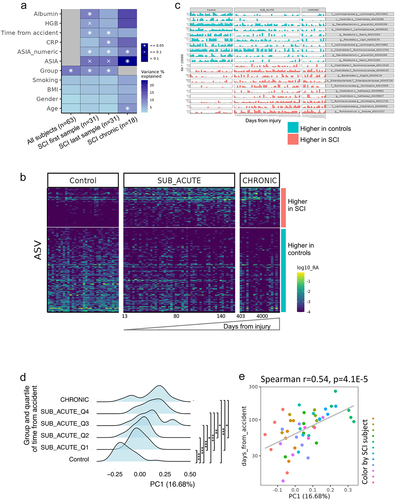
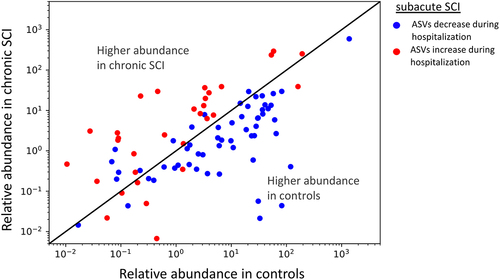
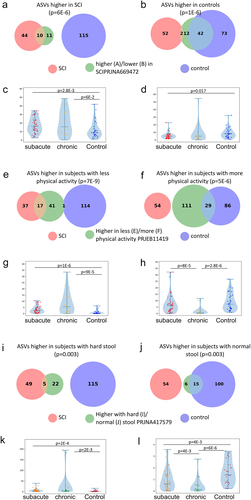
a. Overlap between ASVs significantly higher in SCI in our study (red circle), ASVs significantly higher in healthy controls (blue circle), and ASVs significantly higher in SCI compared to controls in a previously published Chinese cohort
Citation32 (green circle, dbBact annotation ID 7428), showing similar SCI disease signals (Fisher exact’s p-value <6e-6). b. Similarly to 5A (red and blue circles remained as 5A) but comparing to ASVs significantly lower in SCI compared to controls in the mentioned Chinese cohort (green circle, dbBact annotation ID 7427), showing consistent healthy control signal (Fisher exact’s p-value <1e-6). c. Per sample distribution of the weighted dbBact
Citation27 F-score for the term “higher in spinal cord injury” across the three cohort sub-groups (Distribution lines representing min median and max for each subgroup) showing that the bacterial composition of healthy controls from our study are less associated with SCI bacteria identified in the previous Chinese cohort
Citation32 (Horizontal bars denote significant differences between groups with mentioned p-values by nonparametric Mann-Whitney tests). d. Similarly to 5C, but comparing F-score for the term “lower in spinal cord injury”. The bacterial composition of healthy controls from our study is more associated with control bacteria identified in the previous Chinese cohort
Citation32 e-h. Similar to 5A-D but comparing to bacteria positively/negatively associated with self-reported physical activity in the American Gut Project
Citation33(dbBact annotation IDs 1726 and 1725 respectively), showing microbial associations between SCI to lower self-reported physical activity. i-l. Similar to 5A-D but comparing to bacteria positively/negatively associated with normal/hard stool consistency in the Colombian population-based cohort of over-weight adults
Citation28, (dbBact annotation IDs 2918 and 2919 respectively), showing microbial associations between SCI and hard stools.