Figures & data
Figure 1. Schematic outline depicting the intricate pathways of gut-to-brain communication in binge eating disorders. The routes involve metabolic, humoral, endocrine, immune and neuronal pathways. BED and BN were associated with microbiota dysbiosis within the gut. The diverse array metabolites produced by the gut microbiome such as SCFAs, neurotransmitters, neuroactive peptides, can travel through systemic circulation to the brain to modulate host appetite indirectly. SCFAs also possess the capability to stimulate enteroendocrine cells into releasing gut hormones such as PYY, GLP-1 and CCK, which effectively regulate appetite and food intake either through systemic circulation or by acting upon afferent pathway of the vagus nerves. The gut microbiota-derived LPS influences BBB permeability by activating the immune response, ultimately leading to disruption of the host’s energy homeostasis. Finally, the diminished KYNA release from gut microbiota binds to NMDARs and triggers the onset of binge eating syndrome by directly activating the vagus-NTS-PVT neuronal pathway. In CNS, the alteration of various brain nuclei functions has been associated with binge eating behavior, including NTS, hypothalamus (HY), nucleus accumbens (NAc), VTA, Zona incerta (ZI), prefrontal cortex (PFC) and PVT.
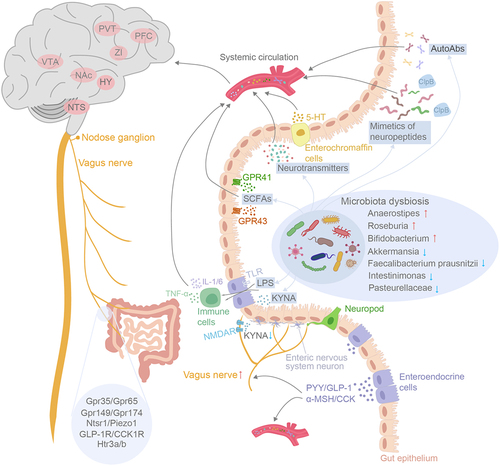
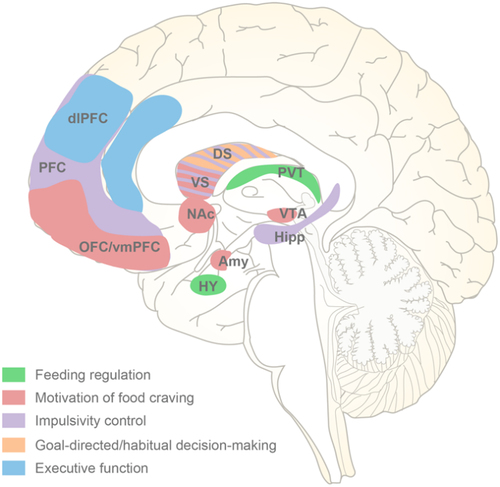
Figure 2. Brain regions in the pathopsychology of binge eating. Based on multiple neuroimaging studies, the central neurological risk factors implicated in binge eating episodes include the dysfunction of five network systems that regulate feeding, motivation, impulsivity control, decision-making and execution. DS, dorsal striatum; VS, ventral striatum.
Data availability statement
This review does not address original research data that need to be publicly deposited.
