Figures & data
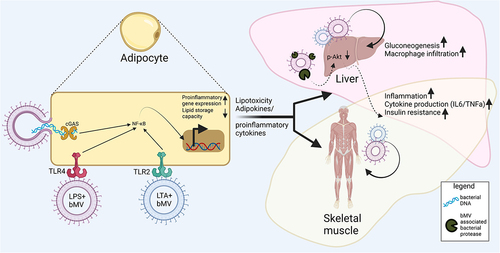
Figure 1. A central role for bacterial membrane vesicles in glucose metabolism and metabolic disease. Illustrating reported effects of gut-derived bMVs on adipose tissue (AT), skeletal muscle tissue and the liver in metabolic disease, with a secondary focus on the consequential signalling effects of AT on skeletal muscle cells and the liver. In mice, gut-derived Pseudomonas panacis bMVs decreased glucose uptake in AT and skeletal muscleCitation23 whereas the translocation of Porphyromonas gingivalis bMVs to the liver reduced glycogen synthesis in response to insulin signalling.Citation25 Interestingly, oral administration of pasteurized Akkermansia municiphila and its bMVs decreased HFD induced measures of inflammation (Interleukin 6 (IL6), Tumor Necrosis Factor alpha (TNFα)) in AT and the liver in mice.Citation24 As master regulator of insulin and glucose homeostasis, AT removes FFAs and TAGs from systemic circulation, thereby preventing lipotoxicity induced insulin resistance in liver and skeletal muscle tissue.Citation6 Via endocrine signalling AT delivers cytokines and adipokines to skeletal muscle tissue and the liver, decreasing insulin sensitivity and fatty acid oxidation in the first, and increasing gluconeogenesis in the latter.Citation88 For the sake of simplicity, the effects of the interaction between the microbiome, its vesicle produce and the cells lining the intestinal barrier on metabolic health, as well as the role of tissue-infiltrating immune cells and crosstalk between skeletal muscle cells, liver and other organs are not illustrated here. TAG: triacylglycerol. FFA: free fatty acids.
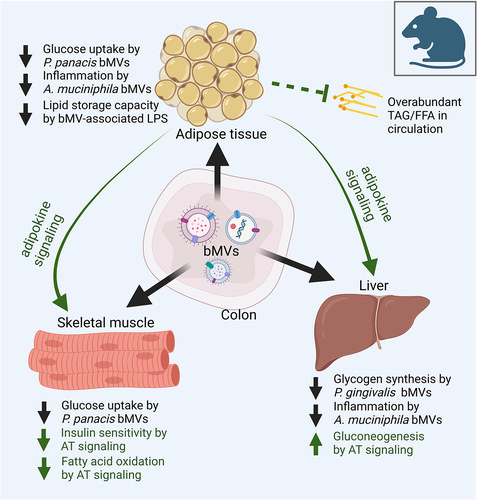
Figure 2. Determinants of bacterial vesiculation and of vesicle characteristics in the large intestine.
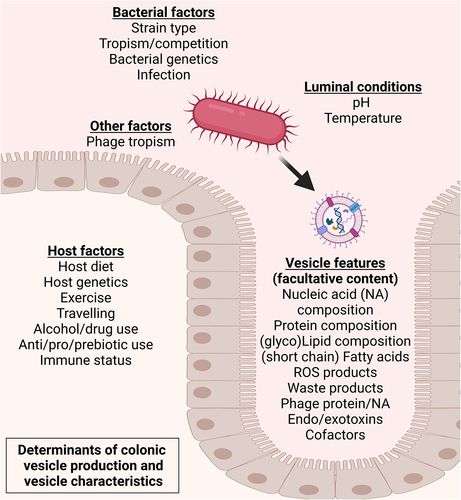
Table 1. Overview of in vivo and in vitro studies investigating involvement of gut-derived bMvs in translocation, (host) inflammation, and/or effects on intestinal barrier function, with potential implications on host metabolic health.
Figure 3. Contextual setting in which the gut microbiota yield a diverse vesicle repertoire interacting with bacterial producer strains and the gut epithelium. Vesicles exiting the intestinal lumen is reported to occur through transcellular migration and through (vesicle induced) disruption of junction proteins regulating intestinal barrier function.Citation10 Vesicle-associated bacterial proteases such as B. fragilis fragolysinsCitation83,Citation84 or possibly P. gingivalis gingipainsCitation85 have been reported able to disrupt barrier function leading to increased leakage of colonic contents. In the intestinal submucosa the (increased) presence with bMVs could induce polarization of extraintestinal immune cellsCitation64 whereas translocation to vasculature facilitates transport of bMVs to distal metabolically relevant tissues and organs.
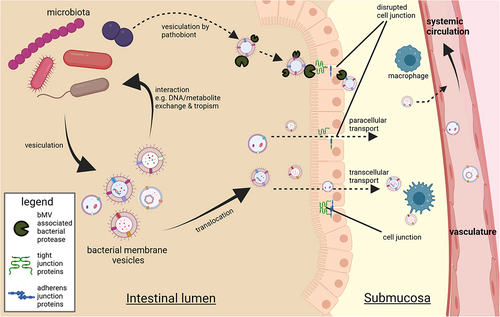
Figure 4. Interactions between bMVs and metabolic tissues. Reported consequences implicated in metabolic disease mostly involve proinflammatory signalling in AT, the liver and skeletal muscle. Mechanisms through which bMVs mediate their biological effects are via activation of pathogen recognition receptors (PPR) such as Toll-like receptors TLR4Citation100 & TLR2Citation101 and cyclic GMP-AMP Synthase (cGAS).Citation95 In addition, bMV associated proteases can dysregulate glucose homeostasis through inhibition of insulin signalling in the liver,Citation25 via mechanisms not fully understood. Upregulated expression of pro-inflammatory genes following PRR activation occurs through translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) to the cell nucleus.Citation107 Proinflammatory signalling in AT is concomitant to a decreased AT lipid storage capacity. A subsequent lipid overflow to non-AT tissues acts in concert with proinflammatory endocrine AT signalling to the liver and skeletal muscle.Citation6