Figures & data
Figure 1. ExPEC reservoir and infection sites.
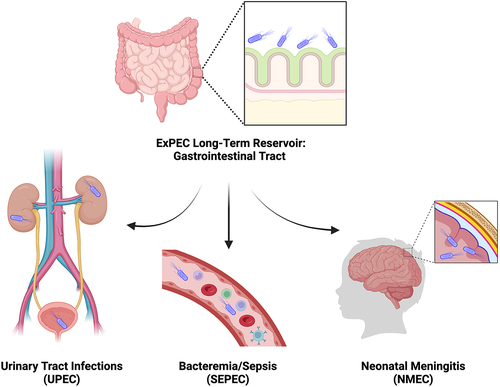
Figure 2. Considerations for antigen and vaccine type selection.
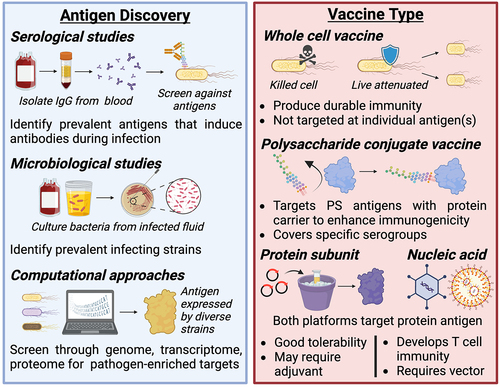
Figure 3. ExPEC vaccine antigen targets.
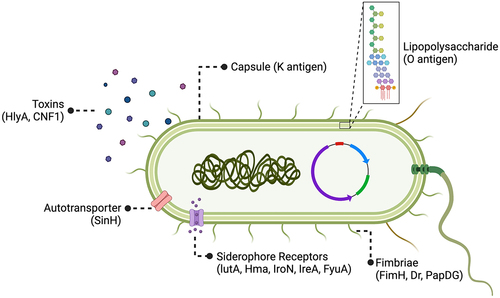
Figure 4. Considerations for adjuvant selection and delivery method.
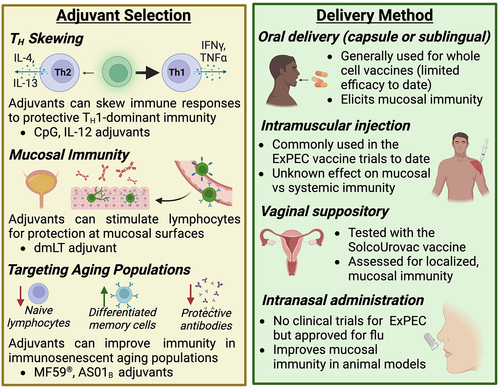
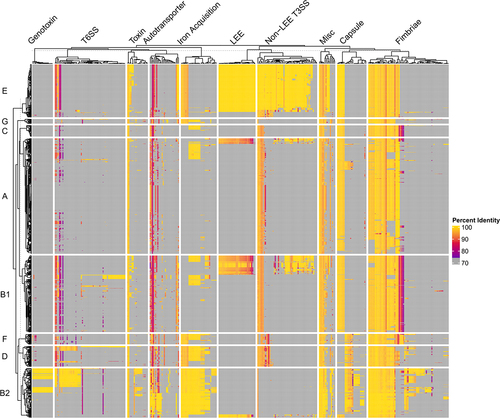
Figure 5. Pan-Virulome of Escherichia coli.
Data availability statement
Data sharing is not applicable to this manuscript because no new data was created.
