Figures & data
Figure 1. Antibiotic treatment successfully depletes the bacteria in the gut of APP-transgenic mice.
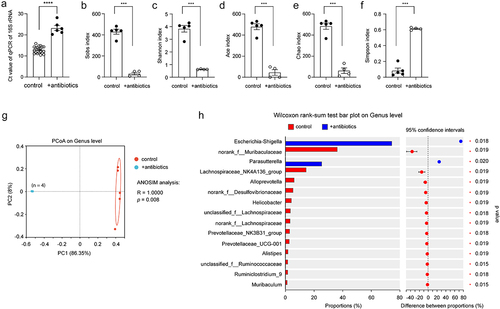
Figure 2. Depletion of gut bacteria reduces Il-17a-expressing CD4-positive lymphocytes.
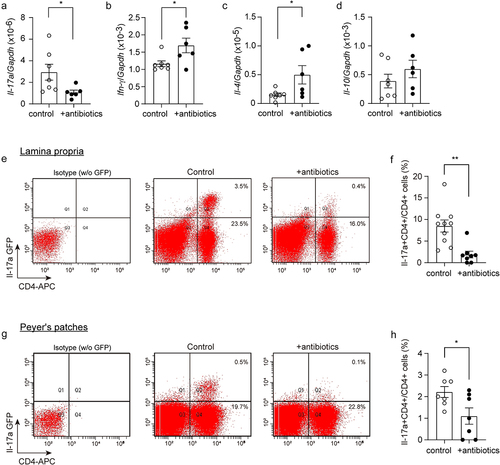
Figure 3. Depletion of gut bacteria reduces bacterial DNA in the brains of both Il-17a-deficient and wild-type APP-transgenic mice.
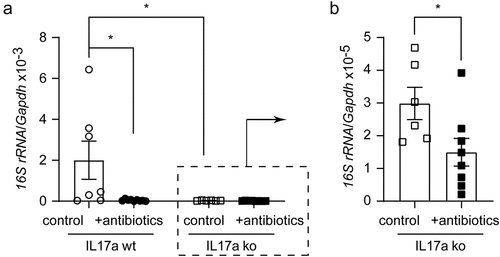
Figure 4. Depletion of gut bacteria reduces inflammatory activation in the brain of Il-17a-wildtype, but not Il17a-deficient APP-transgenic mice.
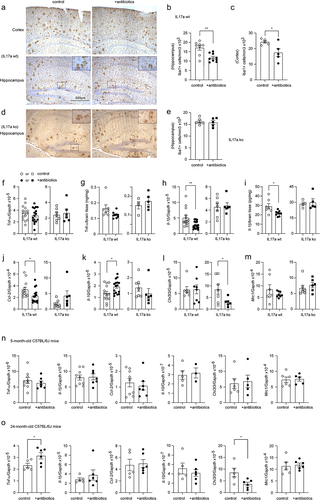
Figure 5. Depletion of gut bacteria inhibits inflammatory activation in microglia in the brain of Il-17a-wildtype, but not Il17a-deficient APP-transgenic mice.
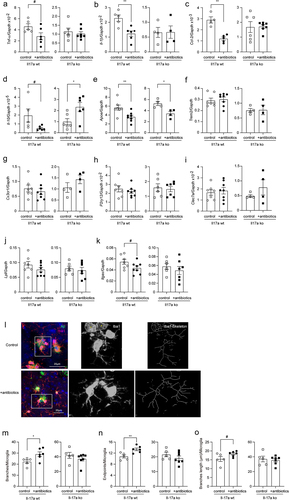
Figure 6. Depletion of gut bacteria reduces Aβ in the brain of Il-17a-wildtype, but not Il17a-deficient APP-transgenic mice.
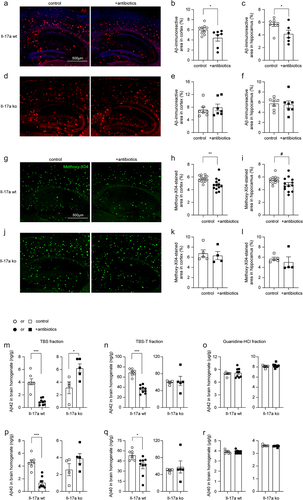
Figure 7. Depletion of gut bacteria reduces β-secretase activity in the brain of Il-17a-wildtype, but not Il17a-deficient APP-transgenic mice.
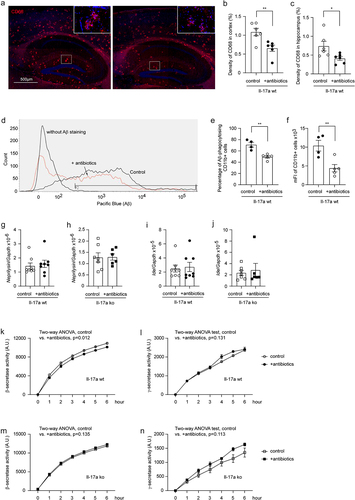
Figure 8. Depletion of gut bacteria increases Abcb1 and Lrp1 expression in the blood-brain-barrier of Il-17a-wildtype, but not Il17a-deficient APP-transgenic mice.
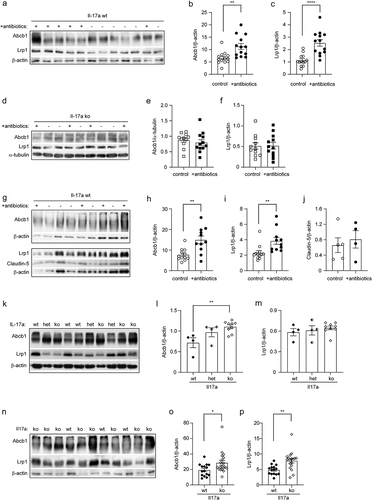
Figure 9. Depletion of gut bacteria increases Arc transcription in the brain of Il-17a-wildtype, but not Il17a-deficient APP-transgenic mice.
Supplementary Figures 220424.docx
Download MS Word (3.9 MB)Data availability statement
Our study has not generated new datasets. All data generated or analyzed during this study are included in this published article and its supplementary information files.
