Figures & data
Table 1. Summary of signals sensed by Lm to modulate virulence.
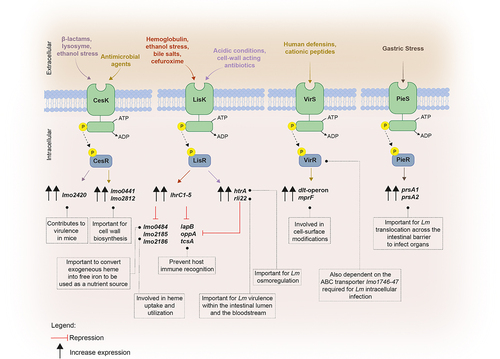
Figure 1. Signalling of Lm virulence gene expression during the infection of the gastrointestinal (GI) tract. (a) The temperature within the mammalian host is key for Lm to modulate the expression of virulence genes. This thermal cue is sensed both by the Lm pleiotropic regulator prfA, whose ribosome binding region becomes accessible enabling the translation of downstream mRNA,Citation25 and by the anti-repressor GmaR that suffers a conformational shift, allowing the expression of MogR and consequently the repression of genes involved in flagellum biosynthesis. Moreover, the Anti0677 is capable of inducing the transcription of mogR by generating a longer anti0677 transcript, positioned on the opposite strand of the flagellum operon coding sequence (fliN, fliP and fliQ).Citation41 within the gastrointestinal tract, Lm encounters bile salts. It uses Bsh, which is crucial for Lm resistance to bile and virulence, to catalyze the hydrolysis of GDCA- and TDCA-conjugated bile salts into free cholic acids, that are exported by the MdrT efflux pump of the bacterium.Citation66 Consequently, in the presence of cholic acids, the bile sensor BrtA, which controls mdrT, is no longer able to repress Lm MdrT.Citation67 (b) the ability of Lm to internalize intestinal epithelial cells relies, in part, on its capacity to form biofilms at the surface of the intestinal villi, which is dependent on MouR that positively regulates the agr operon by binding to its promoter region.Citation103 AgrD is processed by AgrB into a mature AIP, that is subsequently secreted to the extracellular medium. The secreted AIP is then recognized by the sensor kinase AgrC present within the neighboring bacteria, triggering a phosphorylation cascade that culminates in the activation of the response regulator AgrA. In turn, AgrA induces the auto-activation circuit. Therefore, the Agr system induces chitinase activity by the down-regulation of the ncRNA lhrA, which blocks translation by binding itself to chitinase mRNA (chiA). In turn, chitinase represses the inducible nitric oxide synthase (iNOS), known to be part of the immune response.Citation27,Citation104 (c) the activated methyl cycle (AMC) also plays a role in biofilm formation by the generation of the S-ribosylhomocysteine (SHR) by-product, through the processing of S-adenosylmethionine (SAM). Concomitantly, SAM is used to bind and activate SreA riboswitch, that in turn increases the expression of agrD.Citation5,Citation8,Citation10 Simultaneously, the activation of Lm SreA riboswitch by SAM inhibits the prfA transcription in the intestine.Citation3,Citation10 (d) Additionally, within the GI Lm comes across ethanolamine, a metabolite derived from the phospholipids of eukaryotic cell membranes, using it as carbon source to support bacterium growth.Citation105 in presence of ethanolamine, the sensor kinase EutW induces the phosphorylation and activation of the anti-terminator EutV, that together with Rli55 repression, upregulate the expression of the ethanolamine operon eut. The repression of Rli55 is dependent on a B12-riboswitch, located upstream of the eut operon, which in the presence of vitamin B12 blocks the transcription of Rli55 allowing the expression of eut genes. Vitamin B12 is also used as cofactor in propanediol metabolism: in the presence of propanediol, B12 riboswitch ends prematurely the transcription of AsPocR, allowing the transcription of pocR, which is important to activate the pdu and cob operons, respectively involved in propanediol utilization and B12 biosynthesis respectively.Citation106
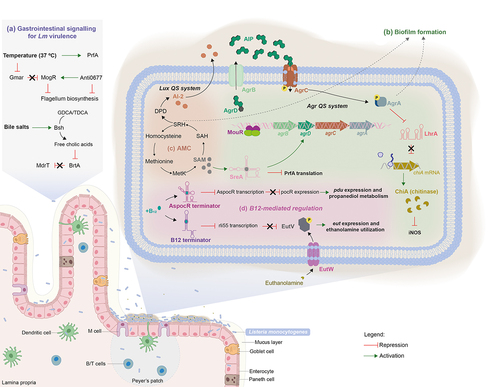
Figure 2. The interplay between host nutritional signals and Lm signaling molecules in virulence. Within the host, Lm imports G6P via the PrfA-regulated permease Hpt, to support its intracellular growth.Citation79 Additionally, Lm uses host L-glutamine whose uptake is dependent on the ABC transporter GlnPQ, to further induce the expression of PrfA-controlled virulence genes. Lm is also capable to synthesize L-glutamine, even not being enough to induce virulence genes, in the presence of ammonia and glutamate by using the glutamate synthase GnlA.Citation77 the expression of prfA is also dependent on glutathione (GSH), which is synthesized from the host cytosol imported L-cysteine, via the glutathione synthase GshF. L-cysteine uptake is mediated by three different transport systems: the substrate-binding CtaP, the Opp transporter and the ABC transporter TcyKLMN. Once synthesized, GSH binds PrfA, inducing a conformational change that enables its binding to the the PrfA box within the promoter regions of PrfA-regulated genes, thereby activating their expression.Citation106 Importantly, prfA-transcription is enhanced in response to the low availability of branched-chain amino-acids (BCAAs) through the binding of CodY to its coding sequence.Citation73 Under high concentrations of BCAAs, CodY binds to isoleucine repressing the expression of both ilv-leu operon and purH gene, involved in BCAA and purine biosynthesis respectively, and also rli60. In contrast, isoleucine-unbound CodY can bind to DNA, allowing its own expression and inducing the expression of prfA and consequently PrfA-regulated genes. When challenged with low BCAA levels rli60 is transcribed forming a ribosome-mediated attenuator that restricts the expression of genes belonging to the ilv-leu operon, in turn increasing Lm virulence through CodY expression.Citation32 CodY-mediated regulation is also dependent on c-di-AMP levels. c-di-AMP is synthesized from two ATP molecules via DacA and degraded by PDEs, PdeA and PgpH, giving rise to pApA. High c-di-AMP concentrations induce the up-regulation of CodY regulon, via CbpB-mediated inhibition of RelA activity.Citation16 This inhibition ensures the maintenance of GTP levels derived from the degradation of c-di-GMP, that induce the expression of CodY.Citation145
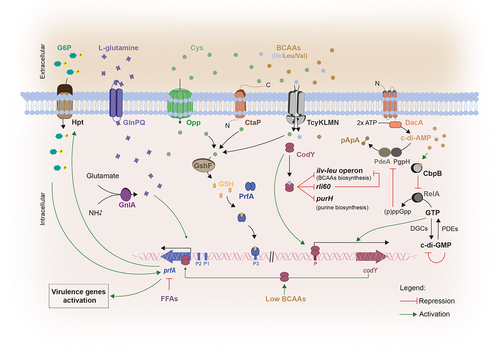
Figure 3. Schematic representation of the two-component transduction systems governing virulence gene expression in Lm.