Figures & data
Figure 1. PL-1C7 monoclonal antibody specifically recognizes the lamin A precursor, prelamin A. (A). Diagram of prelamin A structure and processing. The PL-1C7 monoclonal antibody was raised using as an antigen a synthetic peptide composed of the 12 amino acids spanning the ZMPSTE24 cleavage site in prelamin A (T643-R654), and includes 4 amino acids from the mature lamin A as well as 8 specific prelamin A residues (gray shading). (B). PL-1C7 antibody binding to the carboxyl terminus of prelamin A was confirmed by western blot using a GST_prelamin A V629-M664 fusion protein, right lane; non-fusion protein GST control, left lane. (C). PL-1C7 epitope mapping was done by ELISA immunoassays using a panel of 7 synthetic peptides where wild type amino acids triplets were sequentially replaced by alanine triplets, as well as 2 peptide mimics of ZMPSTE24-generated lamin A fragments: pLA_Mat (G635-Y646) and pLA_frag (L647-S657). Antibody binding was plotted as percentage of binding in relation to the wild type lamin A peptide (pLA_WT). p < 0.005. See also Fig. S1.
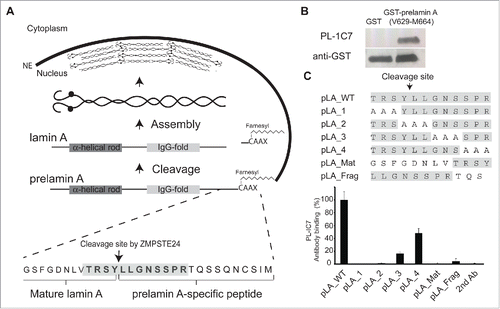
Figure 2. Quantitative detection of prelamin A by intracellular flow cytometry (IFC). Prelamin A is detected by PL-1C7 antibody in Lmna−/− MEFs stably transfected with Doxycycline (Dox)-inducible GFP-Lmna transgene using IFC. (A). Control, non-induced GFP-Lmna MEFs (“x” axis, GFP-fusion; “y” axis, prelamin A signal detected using PL-1C7 antibody). (B). Control GFP-Lmna MEFs after 24 hr Dox treatment stained with secondary antibody only (No PL-1C7). (C). Detection of both precursor and processed Lmna gene products (lamin A) with anti-lamin A/C antibody in GFP-Lmna MEFs after 24 hr Dox treatment. (D). Western blot analysis of GFP-Lmna MEF cells treated with Dox for 24h. GFP signal is present on mature lamin A as well as prelamin A. Prelamin A accumulation was detected using the PL-1C7 antibody. Antibodies against lamin A/C, lamin B and PARP1 were used as controls. (E). Dox-treated GFP-Lmna MEF stained with PL-1C7 antibody (prelamin A). (F). Farnesyl transferase inhibitor (FTinh) induced prelamin A accumulation in GFP-Lmna MEFs detected by IFC using the PL-1C7 antibody (G). Fluorescence geometric median of prelamin A detection using PL-1C7 by IFC after FTihn treatment of GFP-Lmna MEFs. (H). Western blot analysis to detect prelamin A accumulation in Dox induced GFP-Lmna C2C12 myoblasts upon FTinh treatment. Antibodies against lamin A/C and lamin B were used as controls. See also Fig. S2.
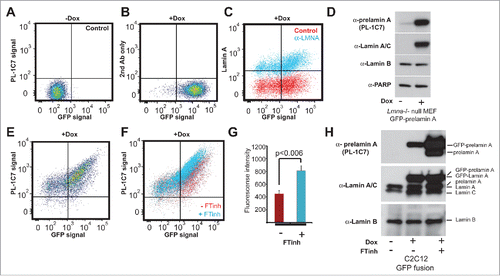
Figure 3. Localized prelamin A detection around the nuclear periphery via immunohistochemical analysis using the PL-1C7 antibody. (A) and (B). Two examples of Dox-treated GFP-Lmna MEF cells stained with prelamin A antibody PL-1C7 (red) and counter-stained with DAPI. GFP signal represents preferentially the mature lamin A, but also the prelamin A fraction due to the GFP tag in the N-terminus (). Dotted boxes show regions where correlation analyses between GFP-lamin A and GFP-prelamin A were performed (dotted line). Signal intensities were normalized to the highest value (100). The signal distribution pattern of the GFP-fusion proteins (GFP signal) representing primarily mature lamin A/C (green line; GFP fusion) is significantly different from the prelamin A distribution pattern detected by the PL-1C7 antibody (red line; prelamin A). (C) and (D). Immunostaining for lamin B, lamin A/C and prelamin A (PL-1C7) in wild-type C2C12 cells. Lamin A/C and prelamin A distribution around the nuclear periphery was analyzed as described in (A) and (B). Scale bar: 5 μm. (E). Simulated measurements to show the utility of the ‘fluctuation index’ metric to assess differences in spatial localization of a target protein relative to a reference protein. The fluctuation index increases as the TEST localization pattern becomes increasingly punctate (Case 1, 2 and 3) relative to a reference pattern. (F). Lamin A/C /lamin B fluctuation index in immunostained C2C12 nucleus (n = 44). (G). Prelamin A/lamin B fluctuation index in immunostained C2C12 nucleus (n = 44).
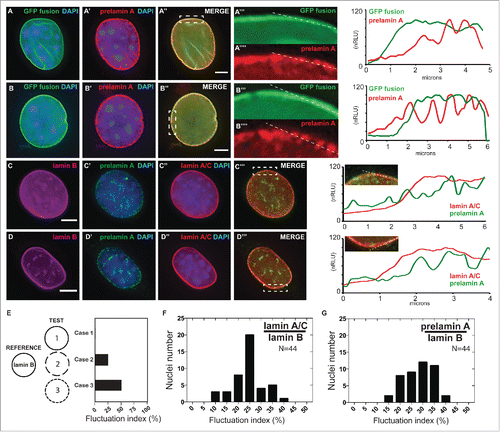
Figure 4. Farnesylation inhibition of endogenous prelamin A causes prelamin A accumulation but not large nucleoplasmic aggregates are not observed. (A) and (B). Dox-induced GFP-Lmna MEFS with and without FTinh (Lonafarnib) treatment were stained with PL-1C7 antibody. (C) and (D). Wild-type MEFs plus and minus FTinh treatment were stained with anti-lamin A/C (green) and anti-prelamin A PL-1C7 (red) antibodies. (E) and (F). Prelamin A and lamin A/C detection in C2C12 myoblasts with and without FTinh treatment stained as in D. (G) and (H). Rhabdomyosarcoma (A-204) cells processed as in E and F. (I) and (J). Co-cultured Lmna−/− (KO) and wild type (WT) MEFs with or without FTinh treatment stained with PL-1C7, anti-lamin A/C and anti-lamin B antibodies. Anti-lamin A/C antibody was used to distinguish KO from WT cells. See also Fig. S3.
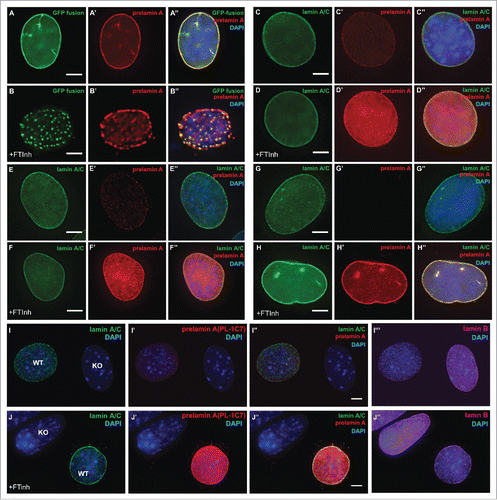
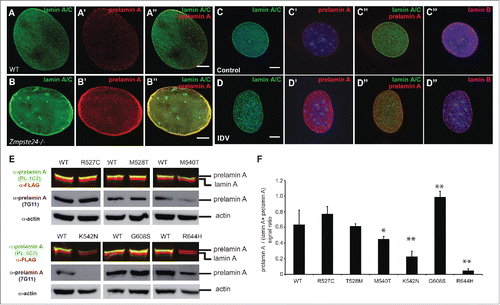
Figure 5. Lack of ZMPSTE24 expression or activity increases prelamin A levels, but laminopathy -associated missense lamin A mutations exert different effects on prelamin A accumulation. (A) and (B). Zmpste24−/− and wild type MEFs were co-stained with anti-lamin A/C (green) and anti-prelamin A (Red) antibodies. Increased prelamin A levels can be observed in the absence of the sequence specific protease. (C) and (D). C2C12 cells were treated with the HIV protease inhibitor indinavir (IDV), which inhibits ZMPSTE24 activity. Cells were co-stained as described in A and including an anti-lamin B antibody as control. (E). Analysis of prelamin A accumulation in laminopathy-associated missense lamin A mutations. Dual infrared immunoblots of total proteins from cells transfected with 3XFLAG-tagged human LMNA constructs containing different laminopathy-associated mutations including: R527C, T528M, M540T, K542N, G608S and R644H. Blot shows the anti-prelamin A PL-1C7 antibody in green (800 nm channel) and a rabbit anti-FLAG antibody in red (700 nm channel). Membranes were re-blotted with anti-prelamin A 7G11 and β-actin antibodies (loading control) and evaluated by chemiluminescence. (F). Quantification of prelamin A levels in laminopathy-associated mutations. Ratio of prelamin A (800 nm channel)/ Total lamin A/prelamin A (700 nm channel) is shown. Values represent the mean +/− SD, * p< 0.005, ** p< 0.001. See also Fig. S4.