Figures & data
Figure 1. Schematic representation of the nuclear envelope. The nuclear envelope consists of an outer (ONM) and an inner nuclear membrane (INM), which are fused at nuclear pore complexes (NPC). The ONM is continuous with the endoplasmic reticulum (ER) and associated with ribosomes as well as nesprins and other proteins linking the nucleus to the cytoskeleton. The nuclear lamina is a proteinaceous network of intermediate filaments underneath the INM that forms extensive contacts with chromatin and INM proteins. The INM harbors multiple nuclear envelope transmembrane (NET) proteins, of which only a few are depicted. LEM domain proteins interact with the chromatin-binding protein BAF and transcription factors, such as SMADs, β-catenin and others, whereas lamin B receptor (LBR) associates with heterochromatin protein 1 (HP1) and directly with modified histones.
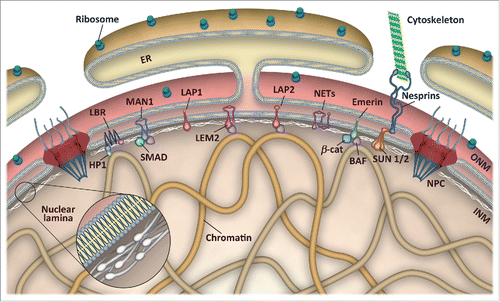
Table 1. Examples of nuclear lamina-related diseases.
Figure 2. Nuclear envelope protein networks controlling nuclear positioning and sensing tension. Trimeric SUN-domain proteins in the INM associate with KASH-domains of nesprins embedded in the ONM and the nuclear lamina to form linker of nucleoskeleton and cytoskeleton (LINC) complexes. In the cytoplasm, nesprins bind a variety of cytoskeletal structures, including filamentous actin, intermediate filaments and microtubules. In addition, LEM-domain proteins are also involved in anchoring of centrosomes to the nuclear envelope via unknown cytoplasmic or ONM proteins [24, 86]. Together, these contacts serve as sensors and transducers of mechanical stimuli from the cytoskeleton to regulate gene transcription as well as to position the nucleus within the cell. The ratio of A- and B-type lamins in the nuclear lamina and phosphorylation of lamins controls nuclear envelope flexibility, which is important during cell migration and tissue deformation.
![Figure 2. Nuclear envelope protein networks controlling nuclear positioning and sensing tension. Trimeric SUN-domain proteins in the INM associate with KASH-domains of nesprins embedded in the ONM and the nuclear lamina to form linker of nucleoskeleton and cytoskeleton (LINC) complexes. In the cytoplasm, nesprins bind a variety of cytoskeletal structures, including filamentous actin, intermediate filaments and microtubules. In addition, LEM-domain proteins are also involved in anchoring of centrosomes to the nuclear envelope via unknown cytoplasmic or ONM proteins [24, 86]. Together, these contacts serve as sensors and transducers of mechanical stimuli from the cytoskeleton to regulate gene transcription as well as to position the nucleus within the cell. The ratio of A- and B-type lamins in the nuclear lamina and phosphorylation of lamins controls nuclear envelope flexibility, which is important during cell migration and tissue deformation.](/cms/asset/c6d1b432-ffbd-44cc-aba2-c840e33e7925/kncl_a_1183848_f0002_oc.gif)
