Figures & data
Figure 1. Schematic depiction of NE rupture sequence during cell migrating through an engineered constriction (gray pillars) mimicking confining interstitial spaces. The nucleus containing NLS-GFP is indicated in green. Right side: close-up of the area indicated in the red dashed box in panel I, containing the leading edge of the nucleus where the NE rupture occurs. (I) The nucleus enters the constriction. (II) Formation of a nuclear membrane bleb at the site of a local defect in the nuclear lamina. (III) The membrane bleb expands and chromatin can enter the bleb through the nuclear lamina opening. (IV) NE rupture results in collapse of the nuclear bleb and spilling of NLS-GFP into the cytoplasm. (V) Following NE repair, NLS-GFP is gradually re-imported into the nucleus. A small ‘lamin scar’ remains at the location of NE rupture. (VI) The cell has passed the constriction and nucleo-cytoplasmic compartmentalization has been restored.
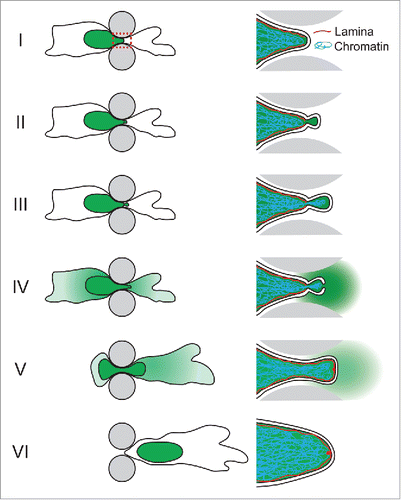
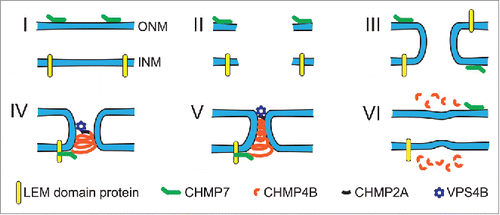
Figure 2. Illustration of the proposed model for ESCRT-mediated NE repair. (I) In the intact NE, membrane-bound CHMP7 is localized to the ER and ONM, whereas LEM-domain proteins are localized to the INM. (II) NE rupture creates exposed hydrophilic domains of the membrane phospholipids and opens the NE lumen to the cytoplasm and nucleoplasm. Given the thermodynamically unfavorable state of open membrane ends, this phase is likely extremely short-lived. (III) The exposed membrane ends of the INM and ONM fuse to each other, resulting in an annular channel connecting the nuclear interior and cytoplasm. The annular channel allows nuclear membrane proteins, including CHMP7 and LEM-domain proteins, to freely diffuse between the INM and ONM without restriction from the nuclear pore complex. Given that LEM-domain proteins interact with chromatin and intranuclear proteins, it is likely that the interaction between LEM-domain proteins and CHMP7 occurs at the INM. (IV) Interaction of CHMP7 with LEM-domain proteins at the annular fusion causes recruitment of CHMP4B to assemble into a spiral of ESCRT-III subunits. CHMP2A, which is incorporated into the ESCRT-III spiral structure, recruits the AAA-ATPase VPS4B. (V) Tightening of the ESCRT-III spiral drives closure of the annular membrane channel and ultimately membrane scission. This process may be mediated through partial disassembly by the recruited VPS4B, or involve sequential assembly of ESCRT-III proteins followed by VPS4B recruitment. (VI) After membrane scission and ESCRT-III complex disassembly have been completed, INM and ONM are separated and NE integrity is restored.