Figures & data
Figure 1. The charge effect is essential for the interaction between the p53 CTD and acidic domain-containing proteins. (A) Schematic diagram of WT/unacetylated p53 and 3 types of p53 mutants. TAD: transactivation domain; PRD: proline-rich domain; DBD: DNA-binding domain; TD: tetramerization domain; CTD: C-terminal domain. (B) Western blot analysis of the interaction between the p53 CTD and acidic domain-containing proteins. In vitro binding assay was performed by incubating GST alone or GST-p53 CTD with purified Flag-tagged acidic domain-containing proteins SET, VPRBP, DAXX or PELP1. The bound components were analyzed by western blot with α-Flag antibody. The purified GST or GST-fusion proteins used in this assay were analyzed in parallel by α-GST antibody
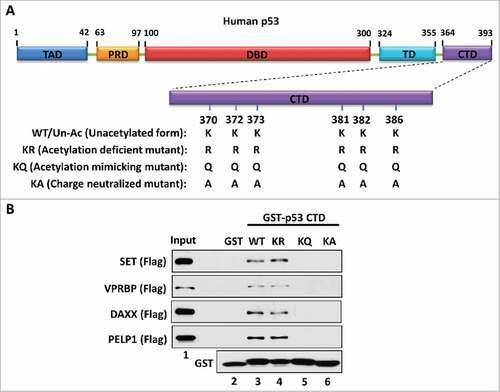
Figure 2. The length of the acidic domain contributes to its interaction with the p53 CTD. (A) Schematic diagram of the full-length and sub-fragments of the SET acidic domain (AD). (B) Western blot analysis of the interaction between p53 and each fragment of the SET acidic domain. In vitro binding assay was performed by incubating purified p53 with purified GST alone or each of the GST-tagged fragments of the SET acidic domain. The binding components were analyzed by western blot with α-p53 antibody. The purified GST or GST-fusion peptide used in this assay was analyzed in parallel by α-GST antibody
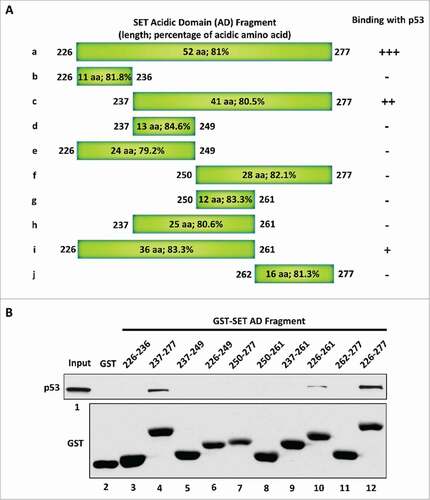
Table 1. A summary of the characteristic features of acidic domain “readers” and other types of acidic amino acid-containing domains. The sequence, length, acidic amino acidic distribution pattern and composition, and the charge of each domain are listed. The acidic amino acids within the sequence of each domain are underlined
Figure 3. The amino acid composition is crucial for acidic domain binding with the p53 CTD. (A,B) Western blot analysis of the interaction between unacetylated p53 CTD and the acidic transactivation domain (TAD). In vitro binding assay was performed by incubating immobilized biotin-conjugated unacetylated p53 CTD with purified GST-p53-TAD (A) or GST-FOXO1-TAD (B), as indicated. The binding components were analyzed by western blot with α-GST antibody. The interaction between p53 CTD and SET acidic domain (AD) was analyzed in parallel as a positive binding control. (C) Schematic diagram of human MDM2 protein. (D) Western blot analysis of the interaction between p53 CTD and MDM2 central acidic domain (CAD). In vitro binding assay was performed by incubating immobilized biotin-conjugated unacetylated or acetylated CTD of p53 with purified GST or GST-MDM2-CAD. The binding components were assayed by western blot with α-GST antibody. (E) Western blot analysis of the interaction between each p53 fragment and the MDM2 central acidic domain. In vitro binding assay was performed by incubating GST or each GST-p53 fragment with purified GFP-Flag-MDM2-CAD. The binding components were assayed by western blot with α-GFP antibody. The purified GST or GST-fusion proteins used in this assay were analyzed in parallel by α-GST antibody
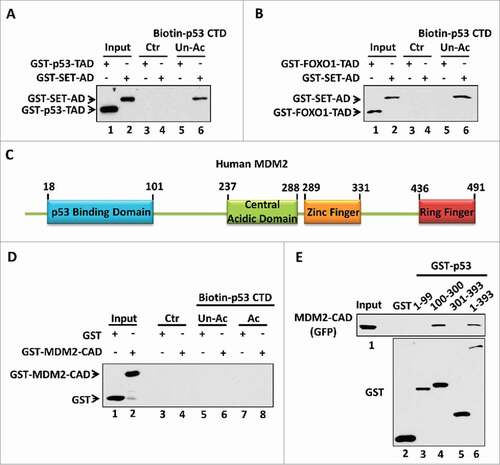
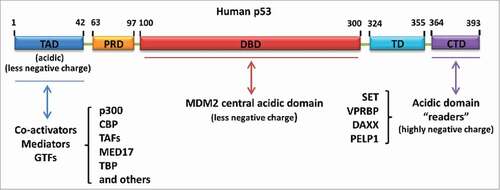
Figure 4. Different types of acidic amino acid-containing domain-mediated regulation of p53. Acidic domain “readers” regulate p53 by interacting with the p53 C-terminus. MDM2 central acidic domain serves as a secondary platform to bind with the p53 DNA-binding domain and promote p53 degradation. The N-terminal transactivation domain of p53 is another acidic amino acid-containing domain responsible for the recruitment of co-activators, mediators or general transcription factors (GTFs) upon p53 activation