Figures & data
Figure 1. Dynamics of the NE during the normal cell-cycle. (a) Interphase NE and ER organization. The interphase ER is continuous the NE and forms an interconnected network of membrane sheets and tubules. (b) In metaphase, the NE is absorbed into the mitotic ER, which is largely excluded from the spindle (purple). (c) Similarly, the mitotic ER remains largely shielded from the anaphase spindle during chromosome segregation. (d-e) (also see section 3 for details) In telophase, segregated chromosome masses recruit membranes to reform the NE. The chromosome regions in contact with the spindle assemble the core NE (thick red lines), whereas the chromosome peripheral regions assemble the non-core NE with NPCs (thick dark green lines). The core membranes abutting the central spindle are termed the ‘inner core’; the core membranes abutting the spindle pole and its microtubules are termed the ‘outer core’. (d) Two hypothetical models for the delivery of (core) membranes into the anaphase/telophase spindle (see section 5 for details): 1. core membrane delivery by direct ER tubule infiltration (red arrows); 2. core membrane delivery by extension of the nascent NE from chromosome periphery/non-core domain (green arrows). (f) (see section 3) In the subsequent interphase, the core NE initially lacking NPCs forms pore-free islands, which progressively assemble NPCs through an inside-out mechanism (purple arrows)
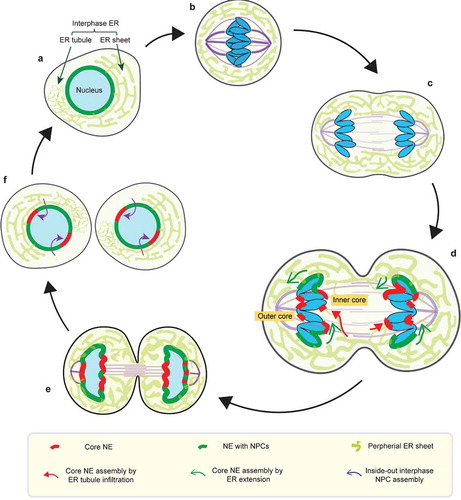
Figure 2. Models for NE/NPC assembly on mis-segregated chromosomes. (a) The chromosome separation checkpoint model: NE and NPC assembly is locally delayed on chromosome regions in proximity to the midzone phosphorylation gradient of Aurora B and CDK1 (see section 5 for details). Under this model, Aurora B activity inhibits NE/NPC assembly by enforcing chromosome condensation. In addition, NE/NPC assembly is inhibited by a gradient of CDK1 activity that mirrors the Aurora B gradient. The chromosome separation checkpoint model was proposed to correct mis-segregated chromosomes. Under this model, mis-segregated lagging chromosomes can be reintegrated through the inner core membrane gaps of the reforming primary nucleus. Accordingly, as the outer core is located furthest to the midzone Aurora B, it is expected to be the first region to assemble NE/NPC as illustrated in the cartoon here. However, at least in some human cell lines, the outer core is often depleted for NPCs during NE assembly (see c). (b) The ‘DNA tether-Aurora B model’: NE/NPC assembly is not delayed on the intact lagging chromosome, but it is specifically delayed on acentric fragments that are connected to the main chromosome mass by DNA tethers. Under this model, the pool of Aurora B coating on DNA tethers inhibits NE/NPC assembly by blocking HP1 recruitment (see section 5 for details). (c) The ‘spindle inhibition model’: on lagging chromosomes, the non-core NE (with NPCs) assembly is inhibited by the mitotic spindle whereas the core NE assembly is either not affected or less affected
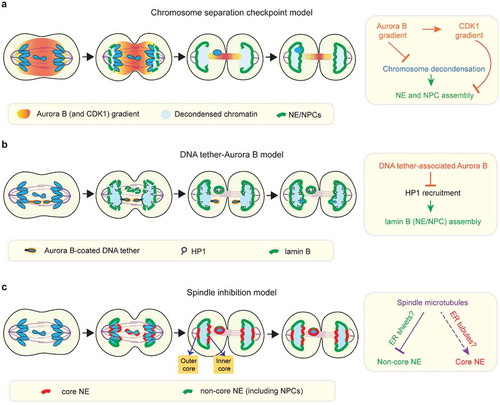
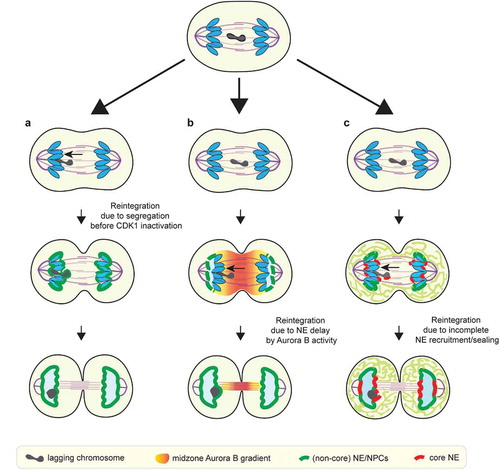
Figure 3. Proposed checkpoint-dependent and checkpoint-independent mechanisms to prevent micronucleation. (a) Lagging chromosome may reintegrate into the main chromosome mass before the main chromosome mass acquires nuclear membrane (checkpoint-independent). (b) Lagging chromosome may reintegrate due to the NE assembly delay induced by the midzone Aurora B activity, as postulated by the chromosome separation checkpoint. (c) Lagging chromosome may integrate due to delayed membrane recruitment/sealing adjacent to the spindle due to the initial ER exclusion from the spindle (checkpoint-independent)