Figures & data
Figure 1. Mechanisms of lncRNAs inducing TMZ resistance. H19 is involved in induction of glioma cell viability and survival through upregulation of MDR1, MRP1, and ABCG2 and activation of NF-κB signaling, responsible for induction of TMZ resistance (Jiang et al. Citation2016; Duan et al. Citation2018). MALAT1 is associated with glioma cell proliferation, viability, and invasion through regulation of ceRNA networks with miRNAs, upregulation of multidrug resistance-related genes, and regulation of ZEB1, which might contribute to glioma TMZ resistance (Chu et al. Citation2003; de Cremoux et al. Citation2007; Pyko et al. Citation2013; Tian et al. Citation2016; Chen et al. Citation2017; Li et al. Citation2017; Cai et al. Citation2018; Dong et al. Citation2019). PVT1 and SBF2-AS1 play oncogenic roles in induction of glioma stemness and cell survival, respectively, through regulation of ceRNA networks, resulting in TMZ resistance in glioma cells (Bazzoli et al. Citation2012; Zhang et al. Citation2019; Gong et al. Citation2021).
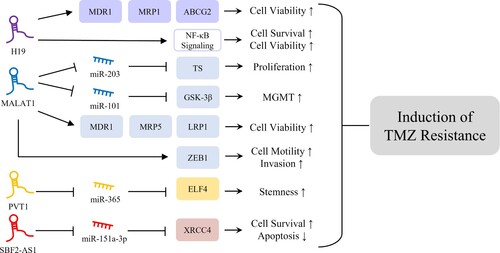
Figure 2. Therapeutic strategies targeting lncRNAs. Downregulation of risky lncRNAs such as AL606760.2 (Li et al. Citation2021), H19 (Chen et al. Citation2020), PVT1 (Chen et al. Citation2020), MALAT1 (Xiong et al. Citation2018), and SBF2-AS1 (Zhang et al. Citation2021) and upregulation of protective lncRNAs such as AC068643.1 (Huang et al. Citation2020), AC079228.1 (Li et al. Citation2021), FAM13A-AS1 (Li et al. Citation2021), HAR1A (Chen et al. Citation2020), DGCR5 (He et al. Citation2020), and WDFY3-AS2 (Wu et al. Citation2018) might help predict prognosis in glioma.