Figures & data
Table 1. Sequences of primers and DNA probes used for standard curves for qPCR.
Table 2. Sequences of nPCR primers.
Figure 1. HHV-6 and HHV-7 viral loads at pre and post-transplantation periods in oral fluids of renal transplant patients (T1: Pre transplantation and without immunosuppression treatment; T2: 15–20 days after transplantation; T3: 45 to 60 days post-transplantation).
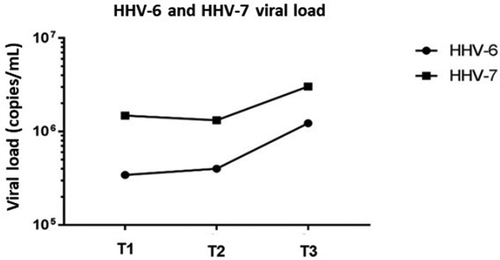
Figure 2. Detection of actively replicating HHV-6 and HHV-7 based on mRNA analysis in oral fluid before and after kidney transplantation. (T1: pre-transplant and without immunosuppressive treatment; T2: 15–20 days after transplantation; T3: 45 to 60 days after transplantation).
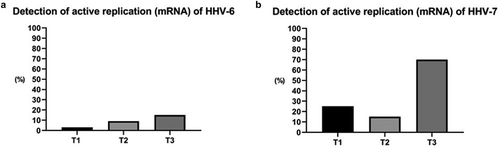
Table 3. Association of HHV-6 and HHV-7 replication with transplant times.
Table 4. Relationship between HHV-6 and HHV-7 DNA levels and mRNA detection.
Figure 3. Correlations between HHV-6 and HHV-7 viral DNA quantities (Spearman correlation: N = 96, Correlation coefficient = 0.781, p < 0.001).
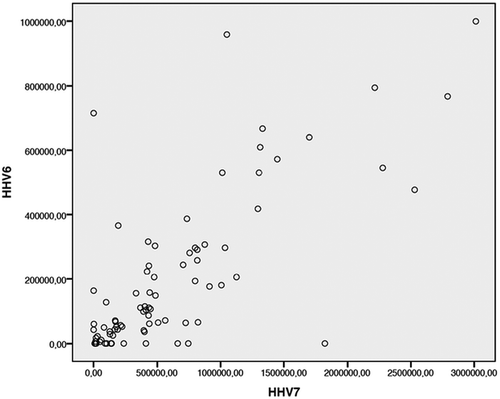
Table 5. Immunosuppressive regimens adopted for the study participants.
