Figures & data
Figure 1. Adherence of MPC polymer to cultured cells.
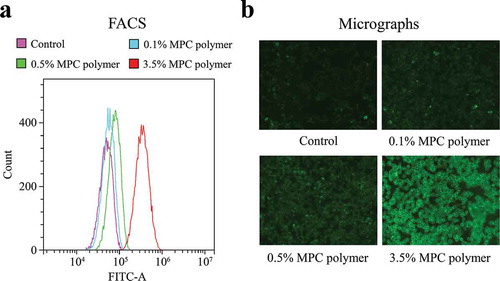
Figure 2. Effects of MPC polymer concentration on the adherence of Spn and NTHi in vitro.
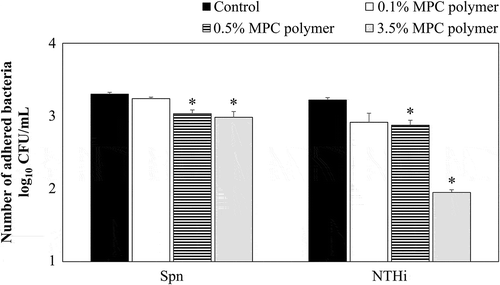
Figure 3. Effects of MPC polymer and PC-KLH on the adherence of Spn and NTHi in vitro.
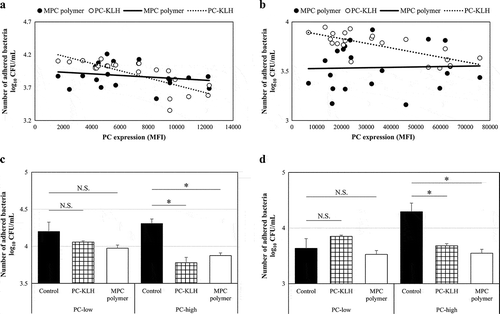
Figure 4. Effects of MPC polymerconcentration on the adherence of Spn and NTHi in vivo.
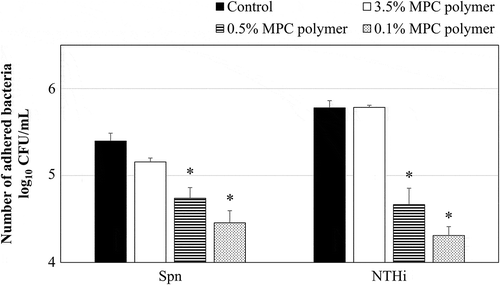
Figure 5. MPC polymer adherence time in vivo.
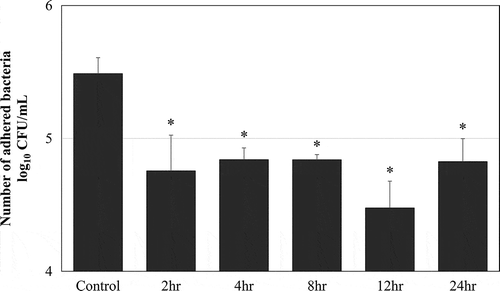
Figure 6. Effects of MPC polymer and PC-KLH on the adherence of Spn and NTHi in vivo.
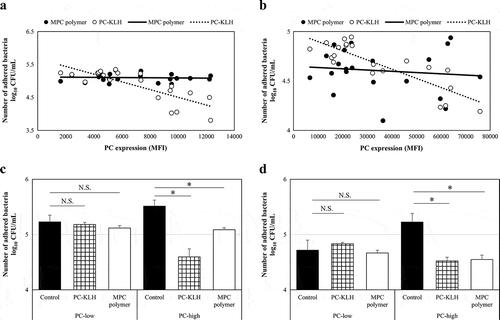
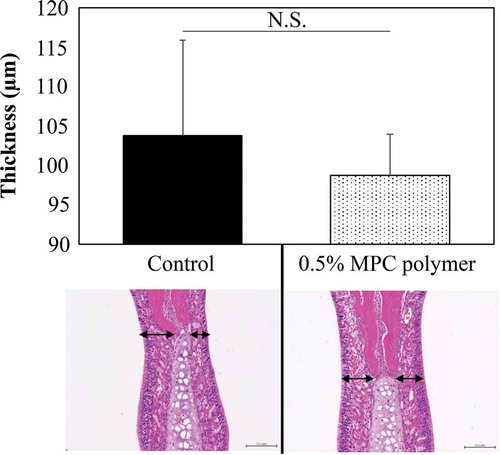
Figure 7. Effect of MPC polymer on nasal mucosa cells.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
