Figures & data
Figure 1. Flow diagram of patients.
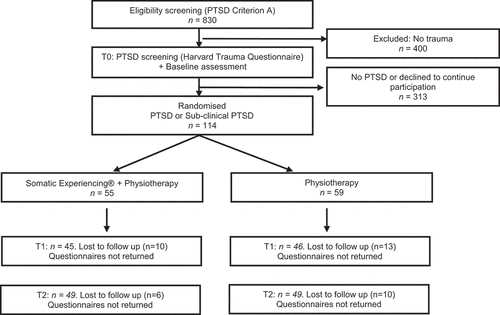
Table 1. Unadjusted means (SD) for outcomes by treatment group and time point.
Table 2. Treatment effects expressed as predicted adjusted mean differences between SE+phys and phys at each time point.
Figure 2. Marginal means and confidence intervals for pain-related disability by treatment group and time point.
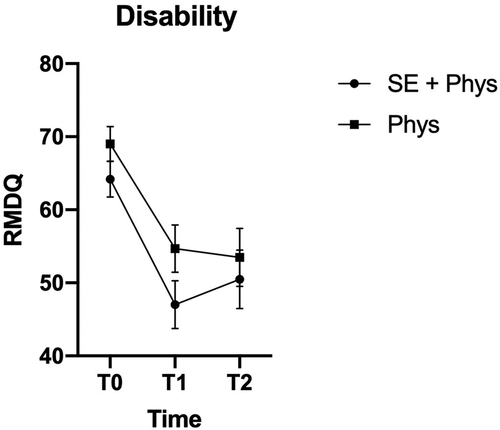
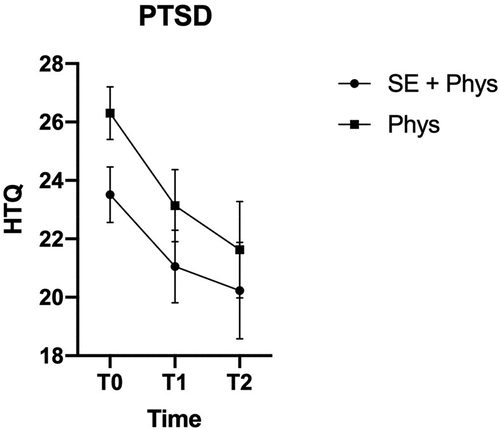
Figure 3. Marginal Means and Confidence Intervals for PTSD by Treatment Group and Time Point