Figures & data
Table 1. Types of EVs.
Figure 1. Biogenesis of EVs.
Exosomes are formed via the endosomal pathway and are released upon fusion of MVBs with the plasma membrane. Microvesicles are generated by the outward budding and fission of the plasma membrane of the donor cells. The apoptotic bodies are large vesicle derived from the apoptotic cells.
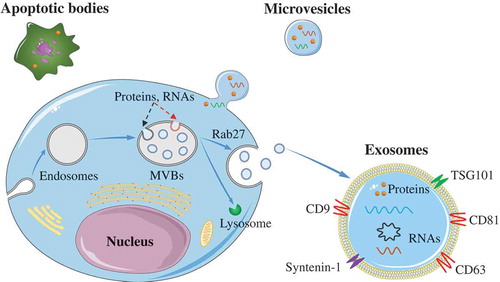
Table 2. Isolation methods for EVs.
Figure 2. Activating inflammation via M1 macrophages.
Adipose tissue derived-EVs or adipocyte derived-EVs could induce insulin resistance in insulin target cells (including adipocyte, hepatocytes, and myocytes) through activating inflammation. The cargos (light blue box) in these EVs were able to promote monocytes or BMMs polarization toward M1 phenotypes. Then, M1 macrophages may induce insulin resistance in target cells through releasing pro-inflammatory cytokines, such as TNF-α, IL-6, IL-1β, and MCP-1. In addition, adipokines carried by the adipose tissue derived-EVs were able to induce insulin resistance.
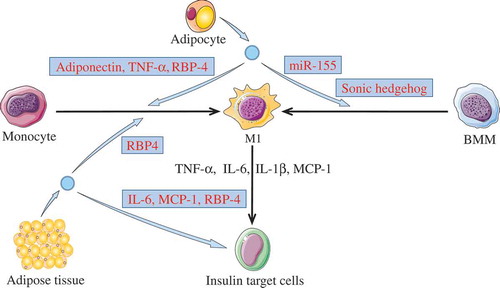
Figure 3. Down-regulating GLUT4 via EVs.
Some EVs can induce insulin resistance by down-regulating GLUT4. miR-155 in M1 macrophage-derived EVs, or miR-27a from the adipocyte-derived EVs can inhibit the expression of GLUT4 via decreasing PPAR-γ. Both miR-883 and miR-450 in EVs from PCs may also affect the expression of GLUT4. In addition, M1 macrophage-released EVs could decrease the GLUT4 translocation from the cytoplasm to the cell surface.
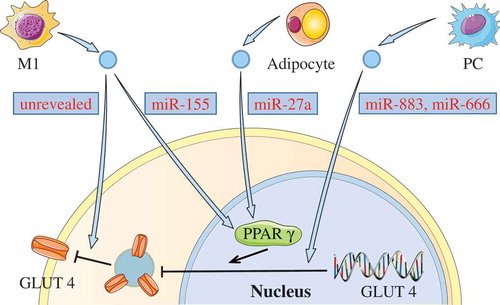
Figure 4. Effect of hepatocyte EVs on insulin receptor.
The β-subunit of insulin receptor was sequentially cleaved by both the calpain 2 and γ-secretase from hepatocyte EVs. The damaged insulin receptor impairs the insulin signalling pathway.
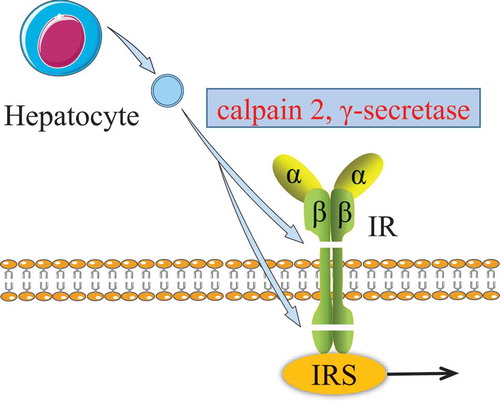
Table 3. the application of EVs in the therapy of T2DM and its complications.
