Figures & data
Figure 1. Acetylcholinesterase activity and particle counts do not correlate. (a) AChE activity (measured within 4 hr after separation) and particle counts of 100 K pelleted EVs from H9 cells measured by (a) NanoSight NS500 (camera setting 12) or (b) ParticleMetrix ZetaView (JHU settings, see Materials and Methods). (c) AChE activity and particle counts of EVs from U937 cells (ZetaView, JHU settings).
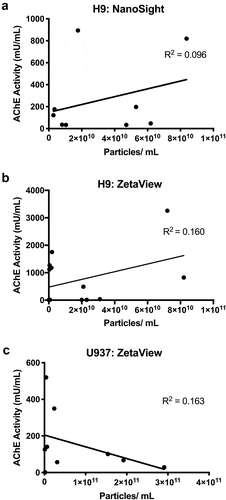
Figure 2. Most AChE activity in serum-containing medium is not pelletable. (a) AChE activity was not detected in serum-free media formulations (AIM Vand RPMI-1640 NCM), but was found at high levels in serum-containing medium (R10 = RPMI 10% FBS NCM). Differential ultracentrifugation of AIM Vand RPMI recovered no activity in differentially centrifuged pellets (2 K, 10 K, 100 K). From serum-containing medium, only a very small fraction of total AChE activity could be recovered by differential centrifugation, and values in complete NCM. (b) After conditioning medium with 72 h culture of PM1 lymphocytic cells, similar amounts of AChE activity as in (a) could be pelleted from serum-containing medium. Comparatively smaller amounts were recovered when cells were switched to AIM V medium.
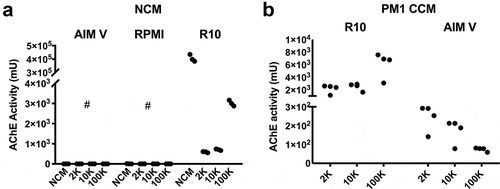
Figure 3. Compared with particle counts, AChE activity is relatively unaffected by conditioning and centrifugation. (a) Scheme of experimental workflow. NCM (R10, shown here, or AIM V, See supplement) was conditioned (CCM) by 72 hr culture of H9 lymphocytic cells. 2 K, 10 K, and 100 K supernatant (S/N) and pellet (P) fractions were obtained. AChE activity and particle counts were measured by activity assay and ZetaView NTA (JHU settings), respectively, for all fractions, and adjusted for total activity (AChE, miliUnits) or particle counts in the original culture volume. (b) Conditioning of R10 and successive depletion of CCM by centrifugation resulted in no significant changes in S/N AChE levels (n = 5). (c) Centrifuge pellets corresponding to the depletion steps in (b) contained only a small amount of total AChE and could not explain minor apparent fluctuations in S/N AChE. (d) Particle counts for the same experiments showed that particles could be depleted progressively from S/N by differential centrifugation (2 K, 10 K, 100 K), reaching significant differences with the 100 K step despite substantial variability. (e) Particles depleted from S/N were substantially recovered in the pellets. (f) Per-particle AChE activity is highest in supernatants, not pellets, and is not enriched in presumed sEV-enriched fractions (representative data of n= 5 experiments). (g) Ratio of per-particle activity of pellets vs corresponding supernatants: the ratio is significantly higher after the low-speed step (2 K) rather than after the presumably sEV-enriching high-speed step (100 K). For all panels, data were compared using ANOVA for matched data and with Tukey’s post-test to correct for multiple comparisons; *: p < 0.05; ***: p < 0.0001. However, for panels B and D, the normalizing group was excluded from analysis because values were set equal to one. Data are from five independent experiments with three technical (measurement) replicates each.
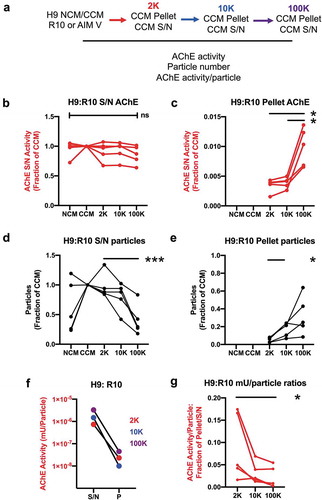
Figure 4. Detection of AChE by Western blot in pellets from different Jurkat cell-conditioned medium (CCM) and non-conditioned medium (NCM). Left panels = representative WB, right panels, quantification of the AChE signals in 3 to 6 independent Western blots. AU = AChE band intensity in a given pellet/sum (AChE band intensity in all 6 pellets). (a) RPMI with 10% FBS. (b) Serum-free TexMACSTM medium. (c) RPMI-10% FBS depleted from serum EVs by overnight ultracentrifugation. (d) RPMI-10% FBS extra-depleted of serum EVs by overnight ultracentrifugation leaving out 5ml above pellet. 2 K, 10 K100 K = pellets recovered from 20ml of CCM or NCM. AChE is clearly detected in non-conditioned serum-containing medium (a), and is only partially depleted by EV depletion (c). (e-g) Representative images of immunogold labelling for AChE of 2 K, 10 K and 100 K pellets recovered from conditioned medium (RPMI-10% extra-depleted of serum EVs). Close-ups of the areas inside white squares are shown. (h) CD63/AChE double stain of 100 k pellets recovered from Jurkat conditioned medium (extra-depleted from FBS).
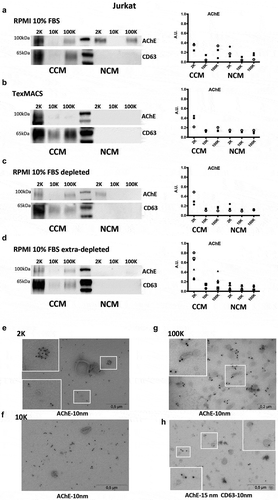
Figure 5. Separation of EVs from soluble proteins by size-exclusion chromatography. 100 K pellets from (extra-depleted) conditioned medium of Jurkat (a-d), and (commercial EV-depleted FBS) conditioned medium of H9 (e-f) and primary RBCs (g-h) were passed through a SEC column, and 14 fractions of 0.5 mL were collected. Western blot for CD63, CD81 (a, e, g) and AChE (a) of fractions 6–14 are shown. (b, f, h) Particle count (NTA) and AChE activity were measured in fractions 6–14 by ZetaView, with settings and measurement parameters as indicated in Materials and Methods for CT (b) or JHU (f, h) laboratories. (c, d) Representative overview and higher magnification images of immunogold labelling for AChE alone (c) or in combination with CD63 (d) of 100 K pellets recovered from Jurkat conditioned medium (extra-depleted from FBS).
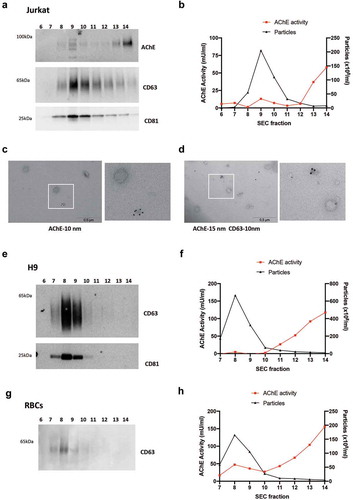
Figure 6. Detection of AChE, EV- and virus-associated proteins in iodixanol velocity gradients of small EVs released by HIV-infected Jurkat cells. (a) Representative Western Blots showing six fractions recovered from iodixanol gradients of the 100 K pellet of Jurkat infected with HIV(NL4-3) virus (extra-depleted medium). Viral p24 protein is recovered in the bottom fractions, cellular CD63 in the middle and bottom fractions, AChE in the top fractions. (b, c) quantification of the AChE and p24 signals in three independent Western blots. AU = band intensity in agiven pellet/sum (band intensity in all 6 pellets).
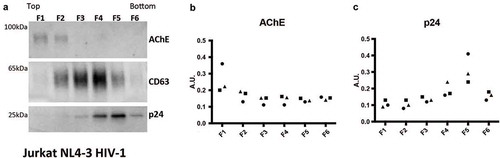
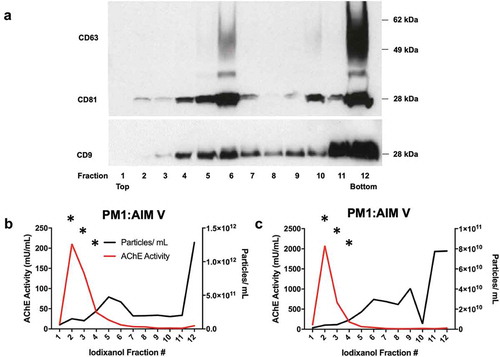
Figure 7. AChE activity and particle counts in iodixanol velocity gradients of small EVs released by HIV-infected PM1 and H9 cells. Twelve fractions were recovered from iodixanol gradients of the 100 K pellet of PM1 cells infected with HIV(BaL) and cultured in commercial EV-depleted FBS medium. (a) Western blot of CD63, CD81, and CD9 in fractions 1–12 (from the experiment also depicted in panel c). (b, c) Particle counts (NTA by ZetaView, with settings and measurement parameters as specified in Materials and Methods for the JHU lab) and AChE activity of two cultures of PM1 cells: values are expressed per mL of final washed and resuspended fraction (1 mL each). Only those points marked by “*” were above the limit of quantitation of the AChE assay (fractions 2–4). Data shown in panels b and c are from 2 independent experiments (with 3 or more technical replicates each).