Figures & data
Table 1. Concentration and cellular origin of extracellular vesicles in human plasma.
Figure 1. Comparison of the thrombin and fibrin generation tests in human plasma. Thrombin generation (TGT; thrombin generation test) 2001 (a) versus fibrin formation (FGT; fibrin generation test) 2018 (b) by extracellular vesicles, Innovin (c, d) or Synthasil (e, f). In both assays, calcium chloride is added at t = 0.
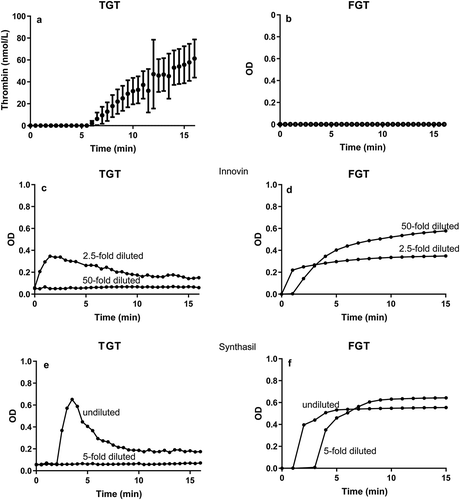
Table 2. Protocols to detect extracellular vesicles and their coagulant activity in 2001 and 2018.
Figure 2. Effect of blood collection and handling on the coagulant properties of human plasma. Platelet-poor plasma (single centrifugation to remove platelets) collected in a siliconized glass tube (a) or plastic tube (b); (c) platelet-depleted plasma (double centrifugation) prepared from blood collected in a plastic tube. For the fibrin generation test (FGT, left), the prepared plasma samples (containing endogenous EVs) were recalcified at t = 0 as described in Methods. For the thrombin generation test (TGT, right), EVs were isolated from the prepared plasma samples and reconstituted in EV-depleted normal plasma as described in Methods, and thrombin generation was initiated by recalcification at t = 0. Please notice that the time scale (X-axis) differs for FGT and TGT. Representative data from one volunteer are shown. In total, six independent experiments were performed.
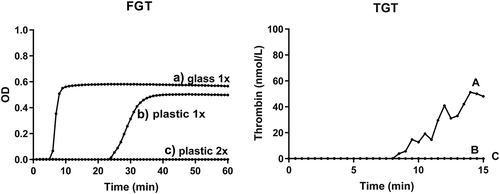
Figure 3. Presence of residual platelets in platelet-poor and platelet-depleted plasma. Platelet-poor plasma was prepared from blood collected in a glass tube (a) or plastic tube (b); (c) platelet-depleted plasma was prepared by double centrifugation from blood collected in a plastic tube.
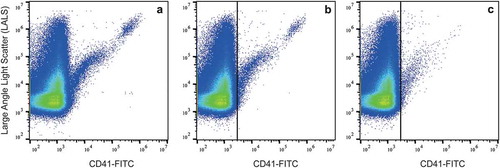
Figure 4. Concentrations of plasma coagulation and fibrinolysis activation markers (a) Concentration of coagulation activation markers prothrombin fragment (F) 1 + 2 and thrombin-antithrombin complexes (TAT) in human plasma; (b) Plasmin generation by EVs in human plasma. Reference ranges are shown in grey.
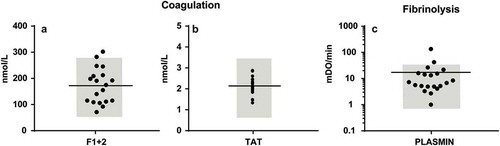
Table 3. Publications presenting information on the coagulant properties of plasma EVs in healthy humans.
