Figures & data
Table 1. Orphan drugs (OD) HTA, pricing, reimbursement, and market access in major European countries.
Table 2. Number of comparable orphan drugs analysed per country.
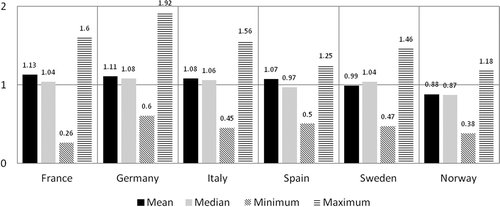
Figure 3. Averaged ratios when the UK reference list was divided into four quartiles based on UK costs. Quartile 1 includes orphan drugs with the highest annual treatment costs in the UK and quartile 4 includes orphan drugs with the cheapest annual treatment costs in the UK.
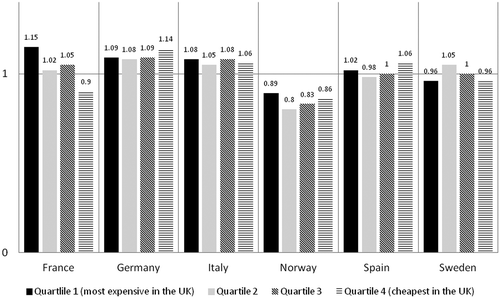
Table 3. Available literature on orphan drug prices in the EU.