Figures & data
Table 1. Disease categories and their subcategories used in recommendations and criteria for biological disease-modifying antirheumatic drug (DMARD) eligibility and the assumptions used to predict their prevalence.
Figure 1. Method used by the population model to calculate populations defined by disease categories. (A) Schematic showing how the model calculates the proportion of the total rheumatoid arthritis (RA) population defined by European League Against Rheumatism (EULAR) recommendations [Disease Activity Score based on 28 joint count (DAS28) > 3.2 and two or more failed conventional synthetic disease-modifying antirheumatic drugs (csDMARDs)]. The model first removes patients with a DAS28 ≤ 3.2 (25% of the total RA population, see for model assumptions) from the total RA population. Next, the model subtracts the RA population with fewer than two failed csDMARDs (56.9%). This leaves 32% of the total RA population that is eligible for bDMARD treatment according to EULAR guidelines. (B) Schematic showing how the model calculates the proportion of a hypothetical country’s RA population defined by national reimbursement guidelines that specify two or more failed csDMARDs and a DAS28 > 5.1. Continuing from the EULAR-defined 32% of the total RA population (as per calculations performed in part A of this figure), the model removes DAS28 ≤ 5.1 patients (80% of the RA population, see for model assumptions) to generate 8.5% of the total population. (1) The higher disease restriction applies to national disease severity criteria that are more stringent than the EULAR recommendations of DAS28 > 3.2.
![Figure 1. Method used by the population model to calculate populations defined by disease categories. (A) Schematic showing how the model calculates the proportion of the total rheumatoid arthritis (RA) population defined by European League Against Rheumatism (EULAR) recommendations [Disease Activity Score based on 28 joint count (DAS28) > 3.2 and two or more failed conventional synthetic disease-modifying antirheumatic drugs (csDMARDs)]. The model first removes patients with a DAS28 ≤ 3.2 (25% of the total RA population, see Table 1 for model assumptions) from the total RA population. Next, the model subtracts the RA population with fewer than two failed csDMARDs (56.9%). This leaves 32% of the total RA population that is eligible for bDMARD treatment according to EULAR guidelines. (B) Schematic showing how the model calculates the proportion of a hypothetical country’s RA population defined by national reimbursement guidelines that specify two or more failed csDMARDs and a DAS28 > 5.1. Continuing from the EULAR-defined 32% of the total RA population (as per calculations performed in part A of this figure), the model removes DAS28 ≤ 5.1 patients (80% of the RA population, see Table 1 for model assumptions) to generate 8.5% of the total population. (1) The higher disease restriction applies to national disease severity criteria that are more stringent than the EULAR recommendations of DAS28 > 3.2.](/cms/asset/50f0c0de-1f7e-4351-bf58-4c35e1129a10/zjma_a_1345580_f0001_oc.jpg)
Table 2. Proportion of countries surveyed with requirements for minimum clinical criteria for biological disease-modifying antirheumatic drug reimbursement.
Figure 2. (A) Changes in national eligibility criteria reported for biological disease-modifying antirheumatic drug (DMARD) reimbursement since 2011. (B) Heat map of countries according to their access scores. (C) Grouping of countries according to low, moderate, and high access composite eligibility scores (see Methods section for definitions of low, moderate, and high). (D) Population sizes of rheumatoid arthritis patients eligible for biological DMARD treatment within national criteria.
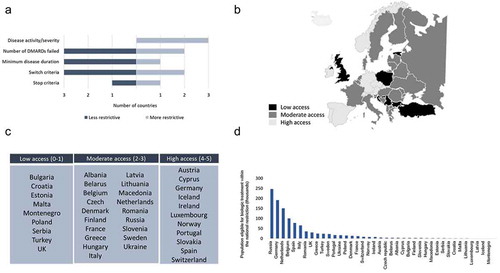
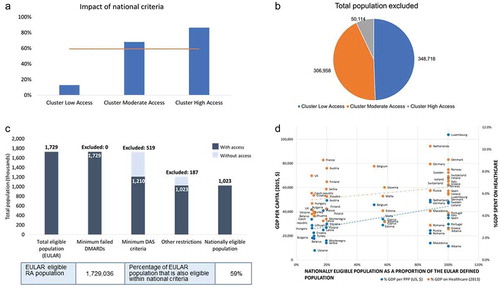
Figure 3. (A) Percentage of European League Against Rheumatism (EULAR)-eligible rheumatoid arthritis (RA) patients in each access group with access to biological disease-modifying antirheumatic drug (bDMARD) treatment on the basis of national criteria. The blue bars denote the eligible proportion of the population in each cluster. The horizontal line indicates the average eligible proportion of the total EULAR-eligible population. (B) Number of EULAR-eligible RA patients in each access group excluded from bDMARD treatment on the basis of national criteria for disease severity and minimum conventional synthetic DMARD treatment failures. (C) Numbers of EULAR-eligible RA patients who are excluded based on national criteria in the European region. In the graph, the dark blue bar denotes the RA population that is eligible according to the given criteria. DAS, Disease Activity Score. (D) The nationally defined RA population as a proportion of the EULAR-defined population plotted against gross domestic product (GDP) per capita in 2015 and % GDP spent on healthcare in 2013. PPP, purchasing power parity. The dotted lines indicate the positive linear correlation.