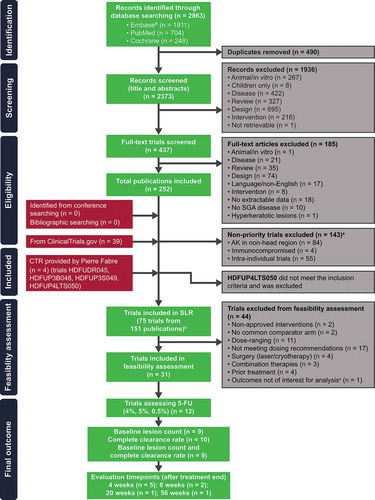
Figures & data
Table 1. Feasibility assessment network for quantitative comparative analyses of 5-FU: exclusion criteria of trials identified from the SLR.
Table 2. Trial and participant characteristics of trials included in the correlation analysis.
Table 3. Complete and partial clearance rates in relation to mean number of lesions at baseline in clinical trials with 5-FU.
Data availability statement
Data derived from public domain resources for published trials. Data available on request from the authors for unpublished trials.
![Figure 2. Correlation between complete clearance rate at 8 weeks and baseline lesion count in trials with 5-FU 4%, 5%, or 0.5% [Citation13–Citation17].](/cms/asset/f9855851-520f-4206-9394-fed5f6839e07/zjma_a_1829884_f0002_b.gif)
