Figures & data
Table 1. Characteristics of randomised, double-blind, parallel-group, placebo-controlled CTs included in the systematic review
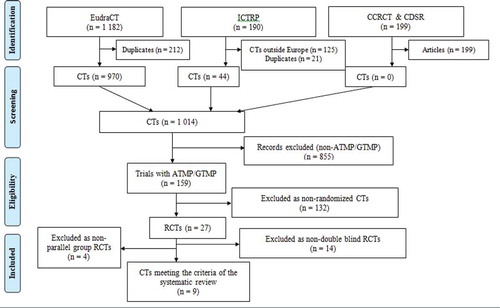
Figure 1. PRISMA Study Flow Diagram. EudraCT: European Union Drug Regulating Authorities Clinical Trials; ICTRP: International Clinical Trials Registry Platform; CCRCT: Cochrane Central Register of Controlled Trials; CDSR: Cochrane Database of Systematic Reviews; CT: clinical trial; ATMP: advanced therapy medicinal product; GTMP: gene therapy medicinal product; RCT: randomized clinical trial; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses