Figures & data
Figure 1. A five-step procedure utilizing a modified Delphi approach was applied to develop and execute the survey.
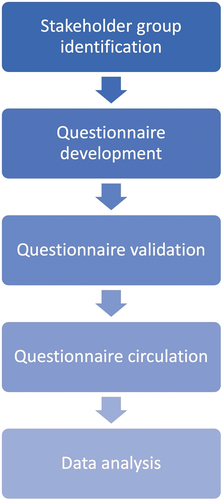
Figure 2. The final questionnaire was developed in two distinct versions: version 1 for self-rating by the ‘key’ stakeholder groups (patients’, clinicians’, regulatory and HTD representatives) and version 2 reflecting the external perception of those 4 ‘key’ stakeholder groups by HTA body representatives, payers, and policy makers. Any other stakeholders that participated in the survey were also provided with version 2.
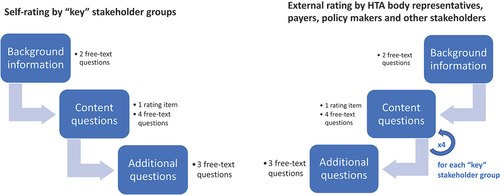
Figure 3. a-b: Background information on submitted questionnaire responses. (a: Number of respondents per stakeholder/collaborator group; b: Number of respondents per country or region, where provided (9 respondents did not provide a country)).
Figure 4. a-d: Box Plots (Mean [x]; Medium; Max; Min; Upper and Lower Quartile) of stakeholder involvement self-rating versus external perception of respective stakeholder/collaborator involvement as rated by HTA bodies; payers; and health policy makers. Scale ranging from 1 to 5, per stakeholder group (a: patients’ representatives; b: clinicians’ representatives; c: regulatory representatives; d: HTD representatives).
![Figure 4. a-d: Box Plots (Mean [x]; Medium; Max; Min; Upper and Lower Quartile) of stakeholder involvement self-rating versus external perception of respective stakeholder/collaborator involvement as rated by HTA bodies; payers; and health policy makers. Scale ranging from 1 to 5, per stakeholder group (a: patients’ representatives; b: clinicians’ representatives; c: regulatory representatives; d: HTD representatives).](/cms/asset/85e85302-5aa0-4261-a02d-dc67d1241010/zjma_a_2217543_f0004_oc.jpg)
Table 1. Key Insights generated within the qualitative part of the questionnaire regarding the content and key challenges for the involvement of the four ‘key’ stakeholder groups.
Table 2. RACI (Responsible/Accountable/Consulted/Informed) Chart for the role definitions of the relevant stakeholder/collaborator groups for the PICO (Population/Intervention/Comparator/Outcomes) categories in the EU HTA procedure as defined in the EU HTA Regulation.
