Figures & data
Figure 1. Illustration of disease and intervention characteristics, and overlaps, which can impact HTA considerations.
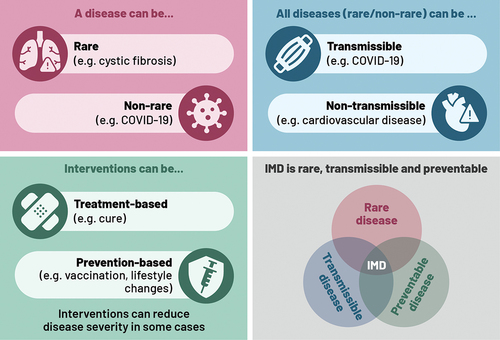
Figure 2. HTA valuation in rare diseases and application to IMD shows the key characteristics of rare diseases and how orphan drugs are assessed by HTA on the left, and the similarities and differences when applied to invasive meningococcal disease (IMD) and vaccination, on the right.Abbreviations: HIV: human immunodeficiency virus; HTA: health technology assessment; IMD: invasive meningococcal disease; QoL: quality of life
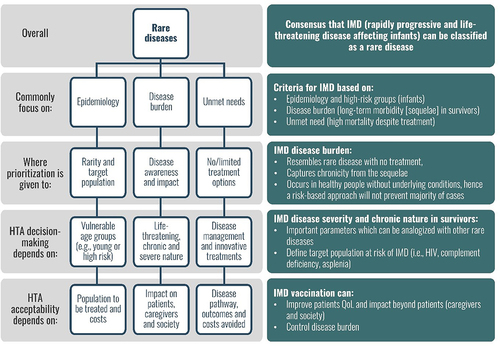
Table 1. Value elements (based on [1, 30]), and experts’ assessment of their inclusion in HTA/CEA, and their applicability to rare, transmissible, and non-transmissible preventable diseases. presents a range of published value elements are assesses which ones are currently included in Health Technology Assessment (HTA)/cost-effectiveness analysis (CEA), and which applicable to rare diseases, transmissible diseases and non-transmissible preventable diseases.
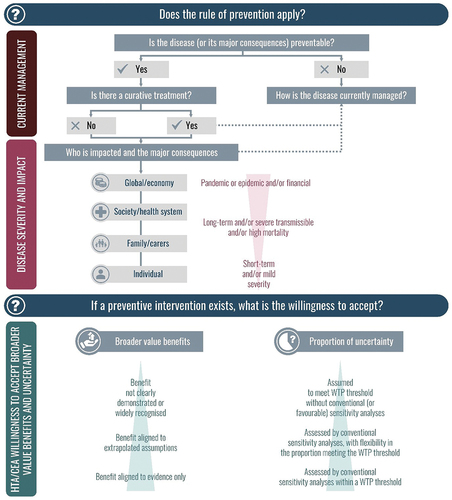
Figure 3. Key considerations when applying the Rule of prevention in HTA – disease severity impact and acceptance of broader benefits and uncertainty. shows the Rule of Prevention framework developed, and highlights the key considerations for its use i.e., disease severity impact and acceptance of broader benefits and uncertainty.Abbreviations: CEA: cost-effectiveness analysis; HTA: health technology assessment; WTP: willingness-to-pay