Figures & data
Figure 1. Flowchart for enrolment of patients in the study. The numbers of patients completing 2 and 3 years of therapy were considered too small for analysis. *Patients used for objective 1; **patients used for objective 2. 1Cause of missing contact: death (1), missing contact information (2); 2side effects registered: general physical discomfort (1), arthralgia (1), headache, fatigue and dyspnoea (1)
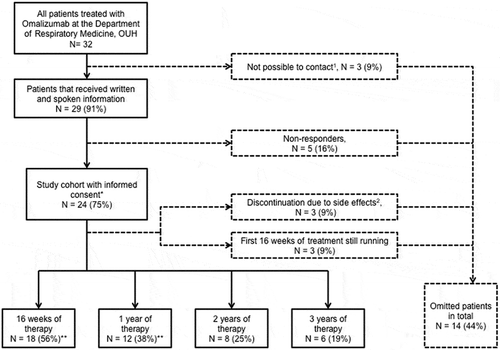
Table 1. Baseline characteristics
Table 2. Fulfilled omalizumab selection criteria
Figure 2. Changes in Asthma Control Test (ACT) score after 16 and 52 weeks of treatment for 17 and 11 patients, respectively. Each line represents a patient and the dotted line represents the mean ACT score. At the 16 and 52 week follow-up the mean ACT score was 17.5 and 19.3, respectively
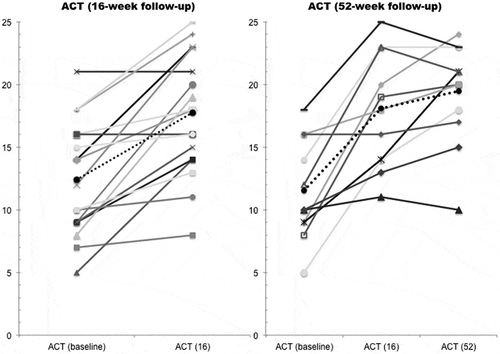
Table 3. Development in mean pulmonary function test (PFT) measurements and mean exacerbation rates for patients completing 52 weeks of treatment
