Figures & data
Table 1. Summary of pharmacokinetic parameters following administration of cromolyn sodium to healthy volunteers in study Part 1; in patients with indolent systemic mastocytosis (ISM) in study Part 2 and in patients with allergic asthma in study Part 3.
Table 2. Summary of adverse events recorded during study Part 1, Part 2 and Part 3.
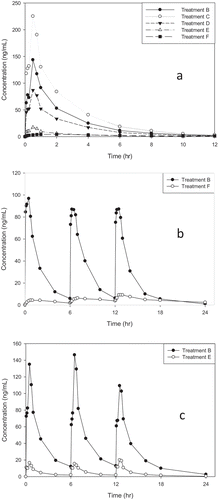
Figure 1. Plasma cromolyn sodium concentrations and mean PK parameters.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
