Figures & data
Table 1. Criteria and incentives for orphan drug in South Korea and three major jurisdictions.
Table 2. Designation and approval status of orphan drugs in South Korea and three major jurisdictions countries (2012–2021).
Figure 1. Approval time, lag time, and designation gap of orphan drugs approved in each jurisdiction.
Note: Based on 169 products designated as orphan drugs in South Korea from 2012 to 2021. We compared the demand time, lag time, and designation gap by country for the products approved in each country. (A) Approval time: South Korea vs. USA, EU, and Japan (P < 0.001). (B) Lag time: South Korea vs. USA and EU (P < 0.001), and South Korea vs. Japan (P < 0.299). (C) Designation gap: South Korea vs. USA, EU (P < 0.001), and South Korea vs. Japan (P = 0.003). (We marked n.s.: not significant, *P < 0.05, **P < 0.01, and ***P < 0.001).
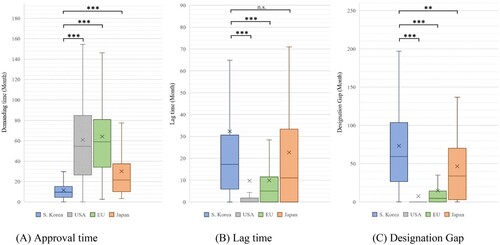
Table 3. Approval time, lag time, and designation gap of orphan drugs approved in each jurisdiction.
Figure 2. Lag time and designation gap of orphan drugs in each jurisdiction, excluding the first approved and designated product.
Note: Based on 169 products designated as orphan drugs in South Korea from 2012 to 2021. We compared the lag time and designation gap by country for products approved in each country, excluding the first-approved and designated products. (A) Lag time: South Korea vs. USA (P = 0.503), the EU (P < 0.001), and Japan (P = 0.903). (B) Designation gap: South Korea vs. USA, EU (P < 0.001), and South Korea vs. Japan (P = 0.011). (We marked n.s.: not significant, *P < 0.05, **P < 0.01, and ***P < 0.001).
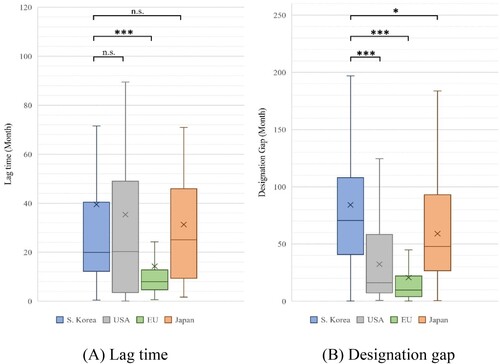
Table 4. Lag time and designation gap of orphan drugs in each jurisdiction, excluding the first approved and designated product.
Figure 3. Approval time, lag time, and designation gap of orphan drugs approved in all jurisdictions.
Note: Based on 169 products designated as orphan drugs in South Korea from 2012 to 2021. We compared the demand time, lag time, and designation gap by country for 45 products approved in all countries. (A) Approval time: South Korea vs. USA, EU, and Japan (P < 0.001). (B) Lag time: South Korea vs. USA and EU (P < 0.001), and South Korea vs. Japan (P = 0.812). (C) Designation gap: South Korea vs. USA, EU (P < 0.001), and South Korea vs. Japan (P = 0.048). (We marked n.s.: not significant, *P < 0.05, **P < 0.01, and ***P < 0.001).
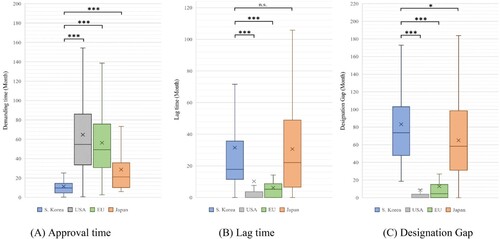
Table 5. Approval time, lag time, and designation gap of orphan drugs approved in all jurisdictions.
Data availability statement
The datasets analysed in the current study are available from the corresponding author upon request.
