Figures & data
Figure 1. Efficiency of different extraction solvent and disperser solvent evaluated for extraction of the four drugs by DLLME. Extraction conditions: sample volume, 5.00 mL; extraction solvent volume, 20 μL; disperser solvent volume, 0.5 mL; room temperature; concentration of each drug, 0.1 μg/mL.
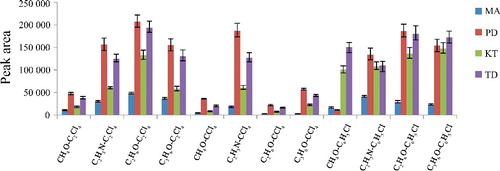
Figure 2. Efficiency of the volume of C2Cl4 evaluated for extraction of the four drugs by DLLME. Extraction conditions: sample volume, 5.00 mL; extraction solvent volume, 5.0, 10.0, 15.0, 20.0, 25.0, 30.0 and 40.0 µL; disperser solvent volume, 0.5 mL; room temperature; concentration of each drug, 0.1 μg/mL.
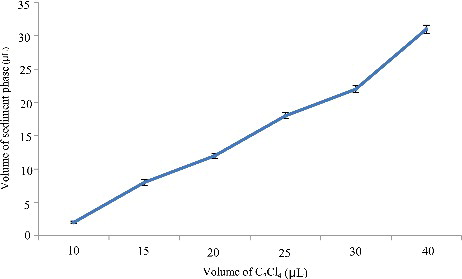
Figure 3. Efficiency of the volume of C2Cl4 on the volume of sediment phase in DLLME. Extraction conditions: sample volume, 5.00 mL; extraction solvent volume, 5, 10.0, 15.0, 20.0, 25.0, 30.0 and 40.0 µL; disperser solvent volume,0.5 mL; room temperature; concentration of each drug, 0.1 μg/mL.
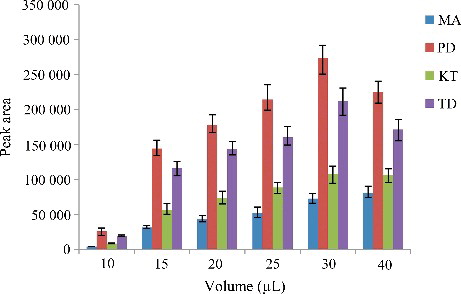
Figure 4. Efficiency of the volume of C2H6O evaluated for extraction of the four drugs by DLLME. Extraction conditions: sample volume, 5.00 mL; extraction solvent volume, 30.0 µL; disperser solvent volume, 0.25, 0.50, 1.00, 1.50 and 2.00 mL; room temperature; concentration of each drug, 0.1 μg/mL.
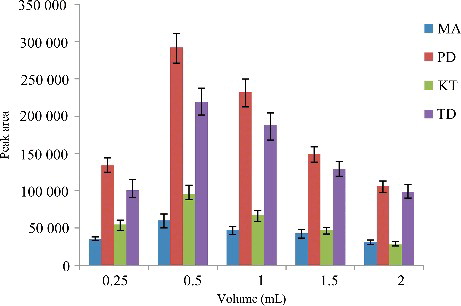
Figure 5. Efficiency of the extraction time evaluated for extraction of the four drugs by DLLME. Extraction conditions: sample volume, 5.00 mL; extraction solvent volume, 30.0 µL; disperser solvent volume, 0.5 mL; room temperature; concentration of each drug, 0.1 μg/mL.
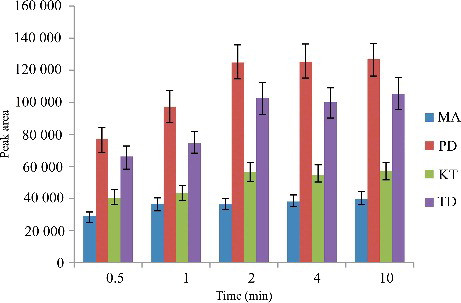
Figure 6. Efficiency of the sample pH value evaluated for extraction of the four drugs by DLLME. Extraction conditions: sample volume, 5.00 mL; extraction solvent volume, 30.0 µL; disperser solvent volume, 0.5 mL; room temperature; concentration of each drug, 0.1 μg/mL.
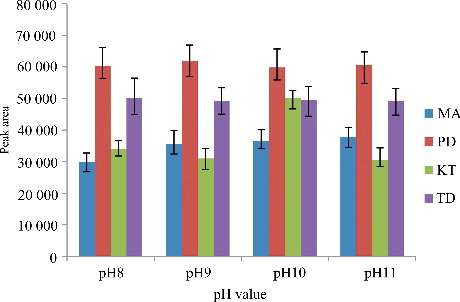
Figure 7. Efficiency of salt evaluated for extraction of the four drugs by DLLME. Extraction conditions: sample volume, 5.00 mL, extraction solvent volume, 30.0 µL; disperser solvent volume, 0.5 mL; room temperature; concentration of each drug, 0.1 μg/mL.
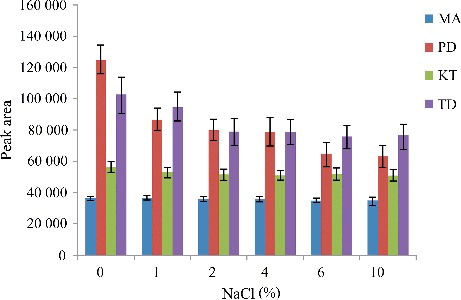
Figure 8. Total ion chromatogram of the four drugs. Extraction conditions: sample volume, 5.00 mL; extraction solvent volume, 30.0 µL; disperser solvent volume, 0.5 mL; room temperature; concentration of each drug, 0.1 μg/mL.
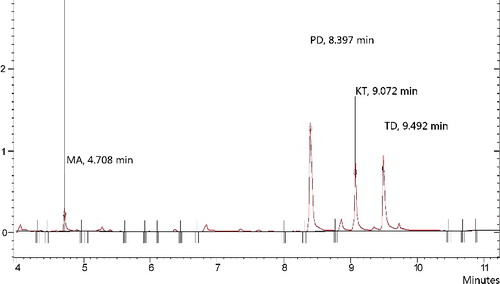
Table 1. Quantification and diagnostic ions used in GC–MS analysis.
Table 2. Quantitative features of the method for the four drugs.
Table 3. Comparison of the method with other studies.
