Figures & data
Figure 1. Symptoms of persimmon anthracnose on Diospyros kaki cv. Wuheshi. A-B, disease lesions on newly-formed twigs. A, large numbers of lesions. B, infection of the whole twig. C, dieback. D, twig canker. E, persimmon tree killed by anthracnose fungus. F, lesions (arrowed) on leaf petioles and veins. G, a fruit lesion on a young fruit. H, premature fruit drop. I, lesions on premature fruits. Note that the cracks are produced longitudinally on fruits.
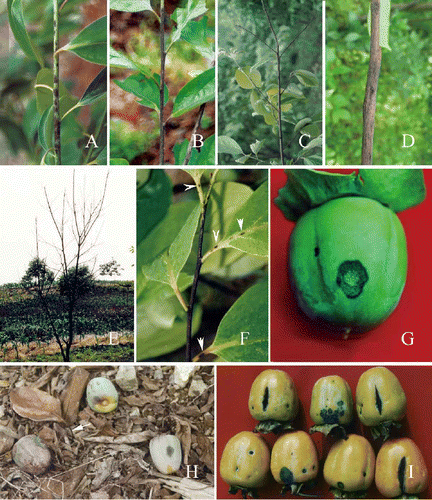
Figure 4. Pathogenicity testing. A-C, Diospyros kaki cv. Wuhesh. Visible symptoms produced after three days after inoculation. B, Yellow-pink conidial masses producing on the central part of a lesion after five days. C, Symptoms after seven days. D, Symptoms on wounded (left) and unwounded (right) banana after five days. E, Symptoms on wounded (left) unwounded (right) marrow after five days.
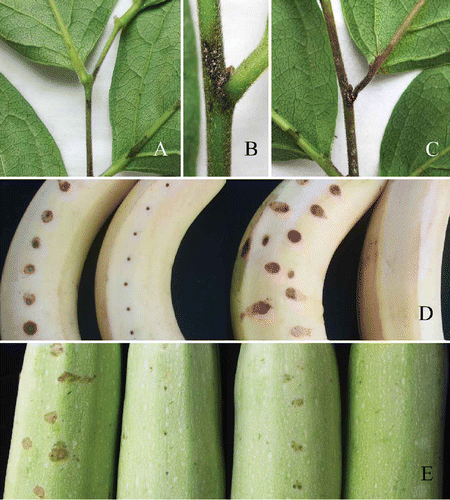
Figure 2. Morphological characteristics of C. horii from Diospyros kaki cv. Wuhesh. A-B, conidia on natural host. A, conidia. B, conidiophores and conidia. C-E, transmission electron micrographs showing conidia. C, straight conidium with obtuse apex and truncate end. Bar = 10 mm. D, a conidium with broken hilum at its base (arrow). E, conidia embedded within and surrounded by the mucilage in acervulus. Bar = 10 μm.
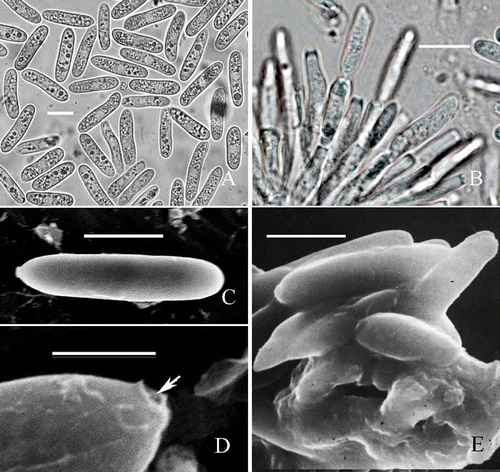
Figure 3. Morphological characteristics of C. horii (from Diospyros kaki cv. Wuhesh). A-C, on PDA. A, view of colony. B, reverse view of colony. C, conidia. D, appressoria formed on polystyrene Petri dish. E, appressoria formed on coverslips. Bar = 10 μm.
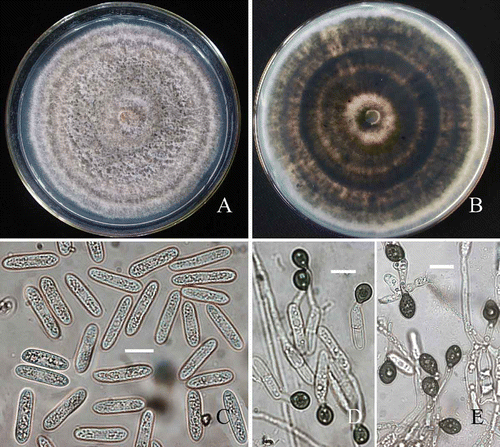
Table 1. Sources of isolates used in this study
Figure 5. Development of fungal infection structures on host cells during interaction of banana with C. horii isolates. A, a primary hypha in an initial infection cell 96 h after inoculation. B, enlarged portion of (A). Note that a primary hypha is separated by an interfacial matrix from the invaginated plasma membrane (arrowhead) surrounding it. C, a primary hypha in an initial infection cell 120 h after inoculation. Note that an enlarged portion of (C) at the right top, in which a primary hypha did not make contact with the host plasma membrane (arrowlead). D, a primary hypha in an initial infection cell 144 h after inoculation. A, appressorium; PH, primary hyphae; IV, infection vesicle; S, septum; Bar = 10 μm.
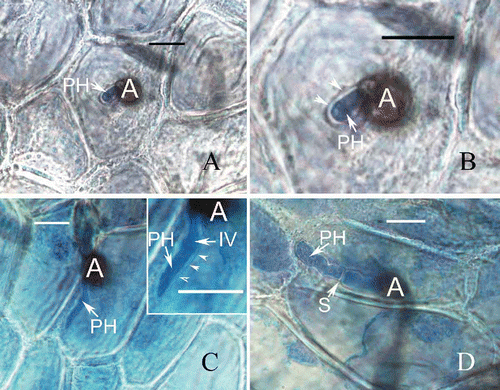
Figure 6. Development of fungal infection structures in host intercellular space and cell walls during interaction of banana with C. horii isolates. A, a primary hypha in intercellular space 120 h after inoculation. Note that a primary hyphae is in close contact with the host cell walls. B, a primary hypha in cell wall 120 h after inoculation. C, a primary hypha in cell wall 132 h after inoculation. Note that swollen primary hypha has caused the host cell wall to curve and rupture (arrowhead).
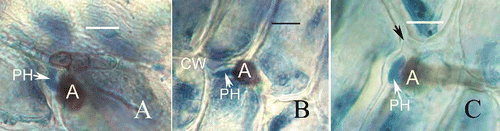
Table 2. Rate of conidial germination in different concentration of a glucose solution
Table 3. Percentage of appressorial formation in different concentrations of a glucose solution
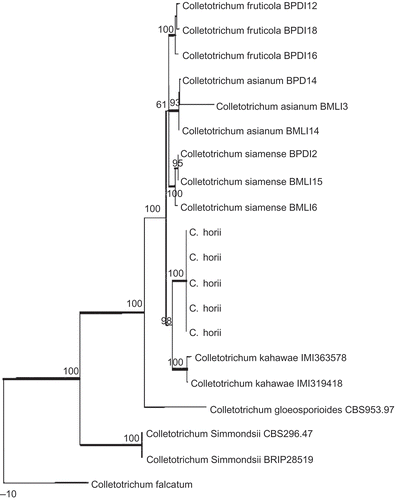
Figure 7. Phylogram generated from parsimony analysis based on combined actin, Bt2, calmodulin, GS and ITS sequences. Data were analysed with random addition sequence, unweighted parsimony and treating gaps as missing data. Bootstrap values ≥50% are shown above or below branches. Thickened branches indicate Bayesian posterior probabilities ≥95%. The tree is rooted with Colletotrichum falcatum.