Figures & data
Figure 1. Chemical structure of fumonisins and analogs. DH4–FB1, tetradehydro fumonisin B1. (A) Structure of fumonism B1 and tetradehydro fumonism B1. (B) Example of possible regio- and sterioisomers of DH4–FB1.
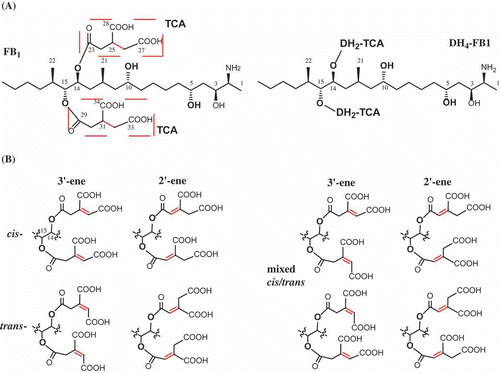
Table 1. Selected HMBC correlations (H→C) for DH4–FB1 and 1H–NMR (500 MHz) and 13C–NMR (125 MHz) spectroscopic data (methanol-d4) for DH4–FB1. δ in ppm. J in Hz.
Figure 2. Isolation and analysis of DH4–FB1 from the FUM7 mutant. (A) HPLC analysis of crude extract; (B) HPLC analysis of the initially purified DH4–FB1; (C) HPLC analysis of the isomerized DH4–FB1; (D) MS analysis of the initially purified DH4–FB1; and (E) MS analysis of the isomerized DH4–FB1. A proposed cis–trans stereoisomerization of DH4–FB1 is included.
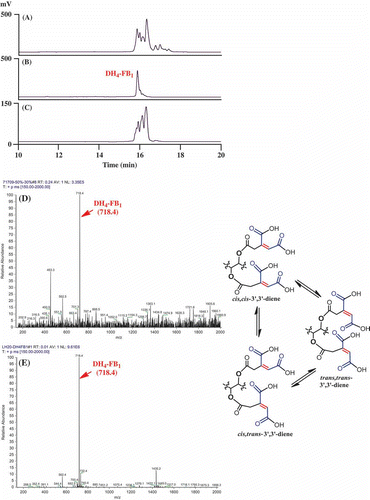
Figure 4. MS analysis of the reaction mixtures containing Fum7p and tetradehydro fumonisins. (A) Standard fumonisins; (B) Fum7p + DH4–FB + NADPH; (C) Fum7p + DH4–FB + NADPH + FeCl2; and (D) Fum7p + DH4–FB + NADH + FeCl2. The peaks corresponding to FB1, FB2/3, as well as the DH4–FB analogs are indicted with arrows. The proposed structure for compounds 1, 2, and 3 are shown in . 1, fumonisin analog with two oxalosuccinate esters; 2, fumonisin analog with one oxalosuccinate ester and one isocitrate ester; and 3, fumonisin analog with two isocitrate esters.
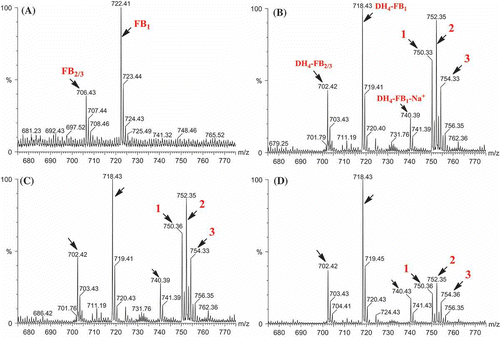
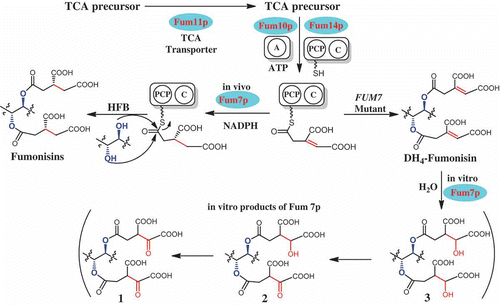
Figure 5. A proposed mechanism for in vivo formation of tricarballylic esters in fumonisin biosynthesis and the observed in vitro activity of Fum7p. TCA, tricarboxylic acid; A, adenylation domain encoded by FUM10; PCP, peptidyl carrier protein domain in the protein encoded by FUM14; C, condensation domain in the protein encoded by FUM14; and HFB, hydrolyzed fumonisins.