Figures & data
Table 1. Secondary metabolites isolated from F. officinalis.
Table 2. Fomitopsins isolated from Fomitopsis species.
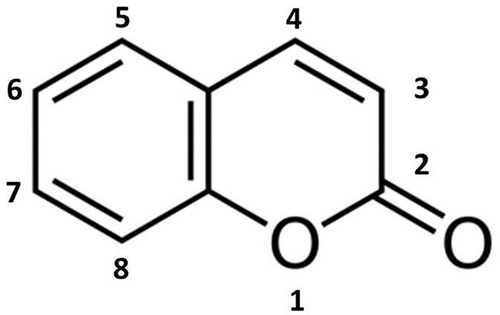
Figure 2. Naturally occurring coumarins (1–2) and newly synthesised analogues (3–4) from F. officinalis. (1) 6-chloro-4-phenyl-2H-chromen-2-one; (2) ethyl 6-chloro-2-oxo-4-phenyl-2H-chromen-3-carboxylate. From Hwang et al. (Citation2013).
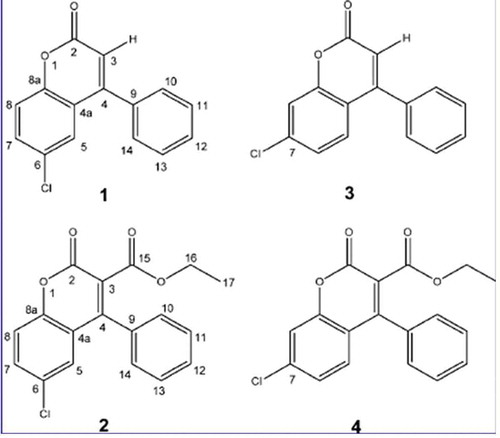
Figure 3. Lanostane or (5S,8R,9S,10R,13R,14S,17R)-4,4,10,13,14-pentamethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,5,6,7,8,9,11,12, 15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene. From: https://pubchem.ncbi.nlm.nih.gov/compound/Lanostane.
![Figure 3. Lanostane or (5S,8R,9S,10R,13R,14S,17R)-4,4,10,13,14-pentamethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,5,6,7,8,9,11,12, 15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene. From: https://pubchem.ncbi.nlm.nih.gov/compound/Lanostane.](/cms/asset/f348bdb4-f4e4-44fd-9412-24cd8787848a/tmyc_a_1536680_f0003_oc.jpg)
Figure 4. Lanostane-type triterpenoids isolated from F. officinalis. (1) 3-[2-(carboxyacetyl)oxy]-12-hydroxy24-methyl-lanost-8,24-dien-26,23-lactone named fomitopsin G; (2) 3-[2-(carboxyacetyl)oxy]-18,23-epoxy-12-hydroxy-24- methyl-lanost-8-en-26,23-lactone named fomitopsin H; (3) 18,23-epoxy-3,12,-dihydroxy-24-methyl-lanost-8-en-26,23-lactone named demalonyl fomitopsin H; (4) ethyl ester derivative of fomitopsin D; (5) fomitopsin F; (6) (25S)-(+)-12α-hydroxy-3αmalonyloxy-24-methyllanosta-8,24(31)-dien-26-oic acid; (7) fomeofficinic acid G; (8) 15α-hydroxy-3-oxo-24-methylenelanosta-7,9(11)-dien-21- oic; (9) fomitopsin C; (10) officimalonic acid A; (11) (3R, 12R, 23S)-3-carboxyacetyloxy-12-hydroxy-24-methyl-7-oxo-lanost-8,24-dien-26,23-lactone, named officimalonic acid B; (12) officimalonic acid C; (13) officimalonic acid D; (14) officimalonic acid E; (15) officimalonic acid F; (16) officimalonic acid G; (17) officimalonic acid H. Modified from Quang et al. (Citation2005), Han et al. (2016) and Naranmandakh et al. (Citation2018).
![Figure 4. Lanostane-type triterpenoids isolated from F. officinalis. (1) 3-[2-(carboxyacetyl)oxy]-12-hydroxy24-methyl-lanost-8,24-dien-26,23-lactone named fomitopsin G; (2) 3-[2-(carboxyacetyl)oxy]-18,23-epoxy-12-hydroxy-24- methyl-lanost-8-en-26,23-lactone named fomitopsin H; (3) 18,23-epoxy-3,12,-dihydroxy-24-methyl-lanost-8-en-26,23-lactone named demalonyl fomitopsin H; (4) ethyl ester derivative of fomitopsin D; (5) fomitopsin F; (6) (25S)-(+)-12α-hydroxy-3αmalonyloxy-24-methyllanosta-8,24(31)-dien-26-oic acid; (7) fomeofficinic acid G; (8) 15α-hydroxy-3-oxo-24-methylenelanosta-7,9(11)-dien-21- oic; (9) fomitopsin C; (10) officimalonic acid A; (11) (3R, 12R, 23S)-3-carboxyacetyloxy-12-hydroxy-24-methyl-7-oxo-lanost-8,24-dien-26,23-lactone, named officimalonic acid B; (12) officimalonic acid C; (13) officimalonic acid D; (14) officimalonic acid E; (15) officimalonic acid F; (16) officimalonic acid G; (17) officimalonic acid H. Modified from Quang et al. (Citation2005), Han et al. (2016) and Naranmandakh et al. (Citation2018).](/cms/asset/166080e7-d9a6-46f6-9376-5ab53e78d8db/tmyc_a_1536680_f0004_b.gif)