Figures & data
Figure 1. Non-canonical DNA secondary structures influencing transcription. (A) Scheme representing the most relevant non-canonical DNA secondary structures and their effects on genome and gene expression. (B) Transcriptional regulation by G-quadruplexes. After transcription onset, transcription bubble generates transiently exposed single-strand segments able to fold as G-quadruplexes. Two putative scenarios are represented (i) G-quadruplexes may form upstream the transcription start site (TSS), causing positive or negative effects on transcription depending on their capability of interfering with RNA Polymerase II or transcription factors binding, recruiting G-quadruplex binding proteins or maintaining an open DNA conformation that facilitates transcription re-initiation. (ii) G-quadruplexes may form downstream the TSS, usually causing positive effects on transcription when located in the coding strand due to favoring transcription re-initiation, or negative effects on transcription when located in the template strand due to stalling the progression of RNA polymerase.
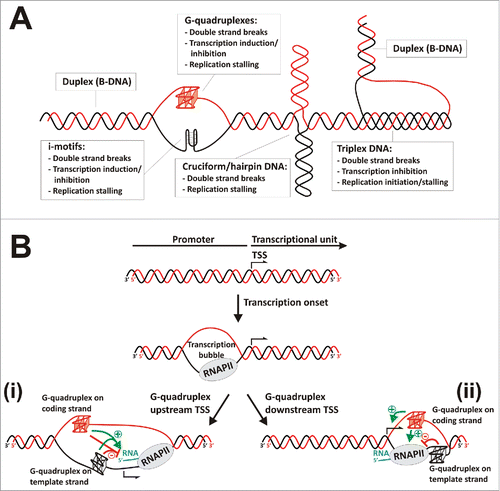
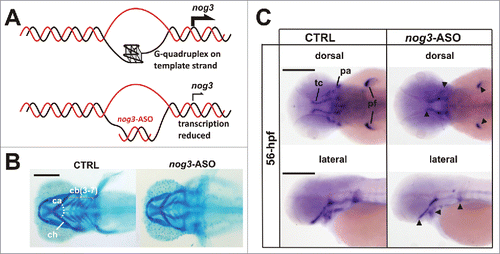
Figure 2. Noggin 3 (nog3), a gene required for proper craniofacial cartilages development, is regulated in vivo by G-quadruplex. (A) Strategy to specifically block G-quadruplex formation using an antisense oligonucleotide (nog3-ASO) microinjected in zebrafish embryos. (B) Alcian blue staining showing craniofacial cartilages (ca, ceratohyal cartilages angle; cb (3–7): ), ceratobranchial cartilages 3 to 7; ch, ceratohyal cartilage) of 4 days post-fertilization (4-dpf) larvae. Compared to controls (CTRL), nog3-ASO microinjected larvae display reduced head structures and abnormal craniofacial cartilage pattern. (C) Lateral and dorsal views of whole-mount in situ hybridizations showing reduced expression of nog3-mRNA in 56 hours post-fertilization (56-hpf) larvae microinjected with nog3-ASO when compared with controls (CTRL). pa, pharyngeal arches; pf, pectoral fin; tc, trabeculae cranii. Scale bars = 200 μm.