Figures & data
Figure 1. Domains and structures of SPT5/NusG in different organisms.
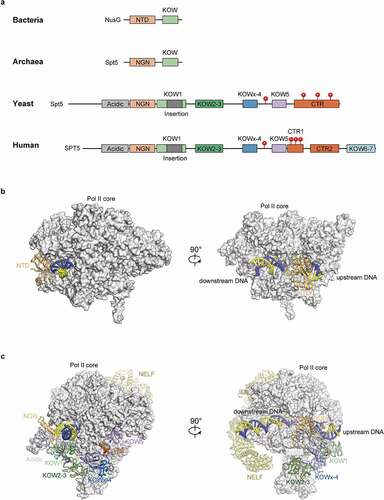
Figure 2. Functions of SPT5 and NELF in stabilizing promoter-proximal Pol II pausing.
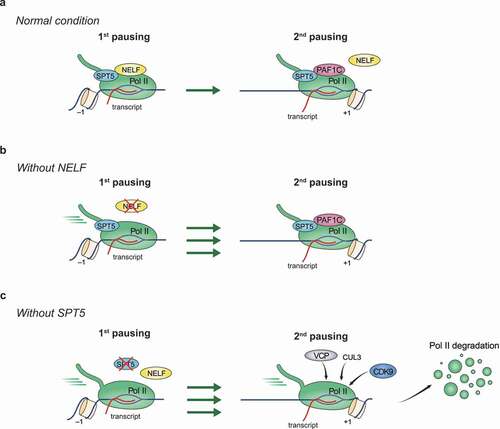
Figure 3. Schematic of SPT5 function in regulating pause release.
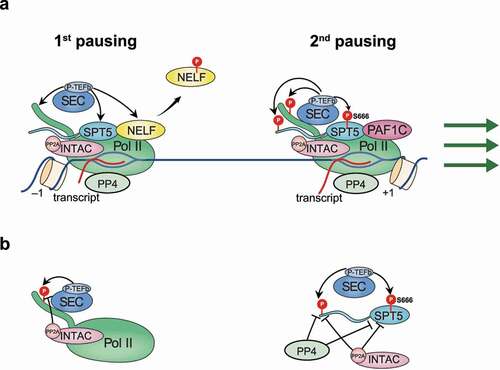
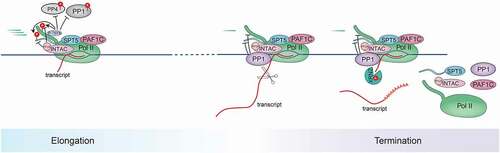
Figure 4. Schematic of SPT5 function in elongation-termination transition.