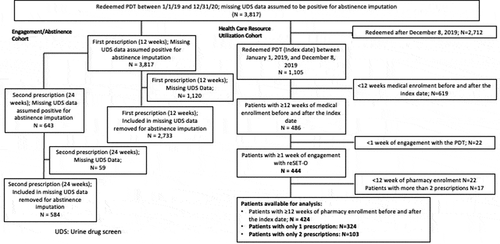
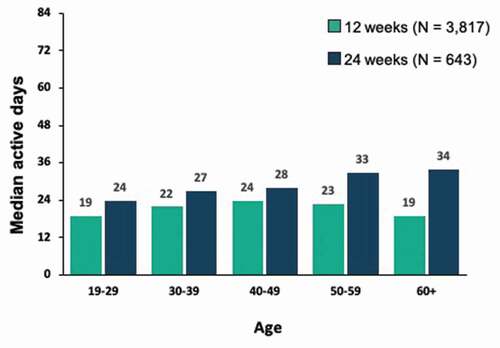
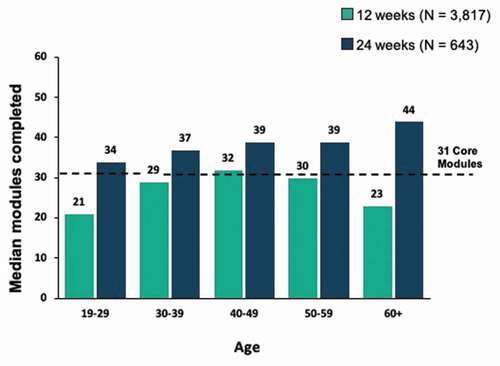
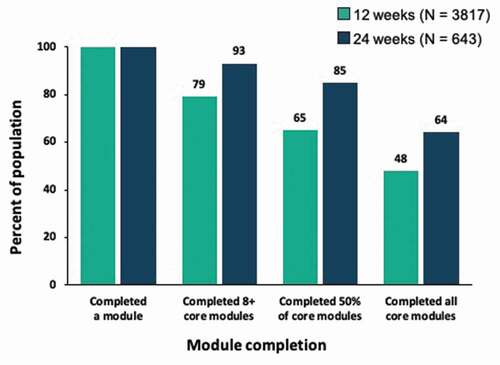
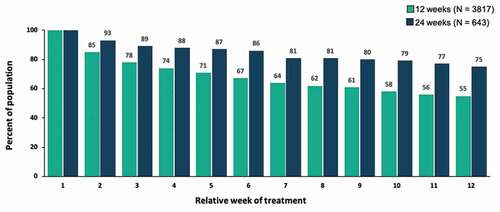
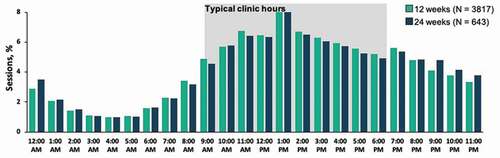
Figures & data
Figure 7. Correlation between module completion and abstinence among patients with 12 weeks vs. 24 weeks of treatment
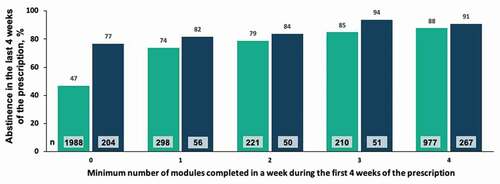
Figure 8. Correlation between module completion and retention among patients with 12 weeks vs. 24 weeks of treatment
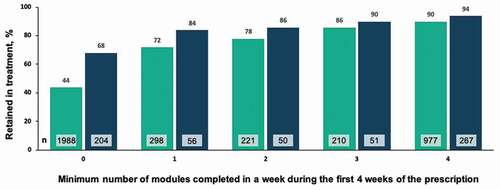
Table 1. Comparison of unique hospital encounters among patients with available insurance claims data