Figures & data
Table 1. Phytochemical analysis and extractive value of different solvent extracts of B. monosperma bark in increasing and decreasing order of solvent polarity.
Table 2. Total phenolic content (as mg gallic acid equivalents (GAE) per g of extract) in different extracts of B. monosperma.
Table 3. Percentage inhibition of DPPH radical in the presence of different concentrations of increasing and decreasing polarity extracts of B. monosperma.
Table 4. Relative reducing power of different concentrations of increasing and decreasing polarity extracts of B. monosperma.
Figure 1. Scavenging of hydroxyl radicals by various extracts of B. monosperma in (a) site specific and (b) non-site specific deoxyribose degradation assay.
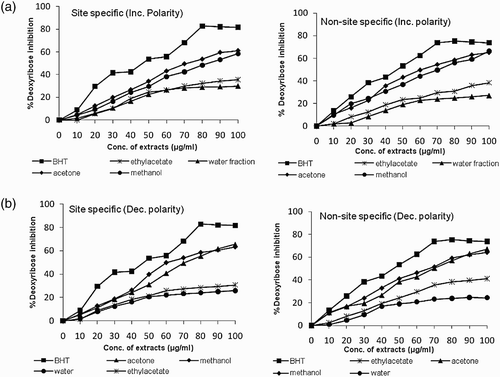
Figure 2. (a) Ferrous ion chelating power and (b) scavenging of the superoxide radical (NBT) by increasing and decreasing solvent polarity extracts of B. monosperma.
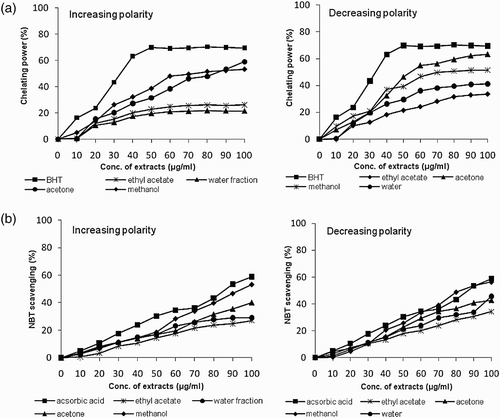
Figure 3. Time course of 20 individual EPR spectra obtained for samples of various extracts of B. monosperma dissolved in DMSO. All sets of 20 EPR spectra of DMPO adducts monitored during the thermal (333 K) decomposition of K2S2O8 in the presence of DMSO extracts were taken for 22 minutes under the same experimental conditions as for reference sample (DMSO, instead of DMSO bark extracts). Extracts concentrations: methanol I.P., acetone I.P. & D.P. (100 mg ml−1) and methanol D.P. (500 mg ml−1).
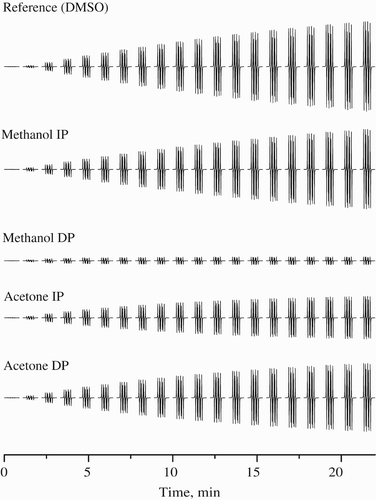
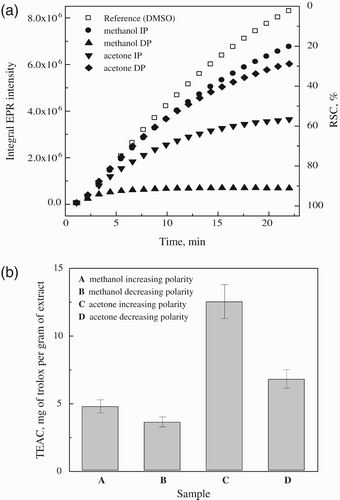
Figure 4. (a) Time course of EPR integral intensities of DMPO adducts recorded during the first 22 minutes of the thermal decomposition of K2S2O8 in the presence of DMSO extracts of B. monosperma bark and (b) radical scavenging capacities equated to actual dry weight of the extracts and expressed as TEAC g−1 of extracts.