Figures & data
Table 1. Phytochemical screening of various extracts of Sargassum polycystum (SP) and Sargassum wightii (SW).
Figure 1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of Sargassum polycystum and Sargassum wightii. (A) DPPH radical scavenging activity of petroleum ether, benzene, ethyl acetate and methanol extracts of S. polycystum. (B) DPPH radical scavenging activity of petroleum ether, benzene, ethyl acetate, methanol and acetone extracts of S. wightii. Butylated hydroxytoluene (BHT) was used as a positive control and absorbance was measured at 517 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant.
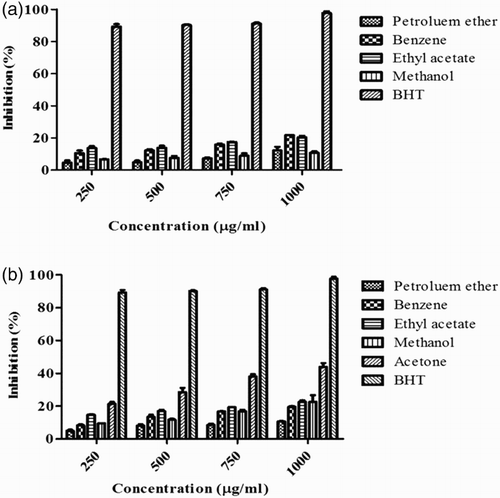
Figure 2. α-Amylase and α-glucosidase inhibitory activity of Sargassum polycystum and Sargassum wightii. (a) In vitro α-amylase inhibitory activity of petroleum ether, benzene, ethyl acetate and methanol extracts of S. polycystum. (b) In vitro α-amylase inhibitory activity of petroleum ether, benzene, ethyl acetate, methanol and acetone extracts of S. wightii. Acarbose was used as a positive control and absorbance was measured at 540 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant. (c) In vitro α-glucosidase inhibitory activity of petroleum ether and methanol extracts of S. polycystum. (d) In vitro α-glucosidase inhibitory activity of petroleum ether, ethyl acetate, methanol and acetone extracts of S. wightii. Acarbose was used as a positive control and absorbance was measured at 405 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant.
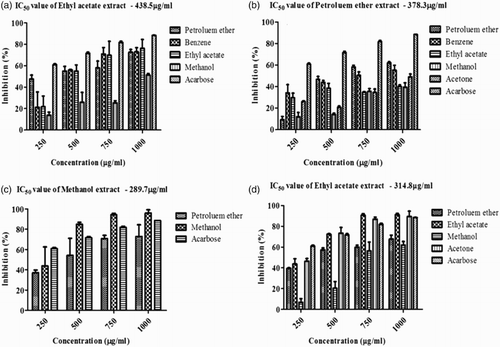
Figure 3. Dipeptidyl peptidase-IV (DPP-IV) inhibitory activity of Sargassum polycystum and Sargassum wightii. (a) DPP-IV inhibitory activity of diprotin A as positive control. (b) DPP-IV inhibitory activity of petroleum ether, benzene, ethyl acetate and methanol extracts of S. polycystum. (c) DPP-IV inhibitory activity of petroleum ether, benzene, ethyl acetate, methanol and acetone extracts of S. wightii. Absorbance was measured at 405 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant.
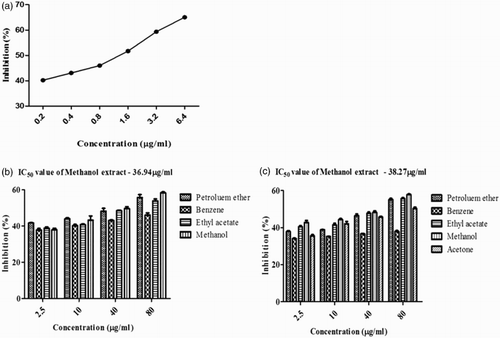
Figure 4. Measurement of cell viability and haemolytic activity of Sargassum polycystum and Sargassum wightii. (a) Cell viability determined by MTT assay. J774 cells were treated with 1000, 750, 500 and 250 µg/ml of ethyl acetate and methanol extracts of S. polycystum (SP), or methanol and petroleum ether extracts of S. wightii (SW). Absorbance was measured at 630 nm. (b) Haemolytic activity of ethyl acetate and methanol extracts of S. polycystum (SP) and methanol and petroleum ether extracts of S. wightii (SW). Absorbance was measured at 630 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant.
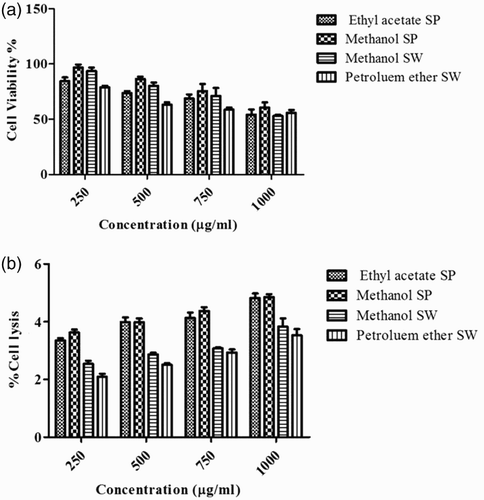
Figure 5. In vitro haemolytic activity, cytotoxicity and DNA fragmentation studies of Sargassum polycystum and Sargassum wightii. (A–E) Microscopic images (40× magnification) of in vitro haemolytic activity of various extracts of S. polycystum and S. wightii: (A) control, erythrocytes treated with normal saline, (B) erythrocytes treated with test extract, S. polycystum ethyl acetate extract (SP EA), (C) S. polycystum methanol extract (SP M), (D) S. wightii methanol extract (SW M), (E) S. wightii petroleum ether extract (SW PE). (F, G) Phase-contrast microscopic images of J774 cells treated with media alone: (F) control and (G) cells treated with test extracts. (H–L) Fluorescent photomicrographs (100× and 40× magnification) of J774 cells treated without seaweed extracts: (H) control, (I) J774 cells treated with S. polycystum ethyl acetate extract (SP EA), (J) J774 cells treated with S. polycystum methanol extract (SP M), (K) J774 cells treated with S. wightii methanol extract (SW M), (L) J774 cells treated with S. wightii petroleum ether extract (SW PE). Each experiment was performed in triplicate (n = 3) and generated similar morphological features.
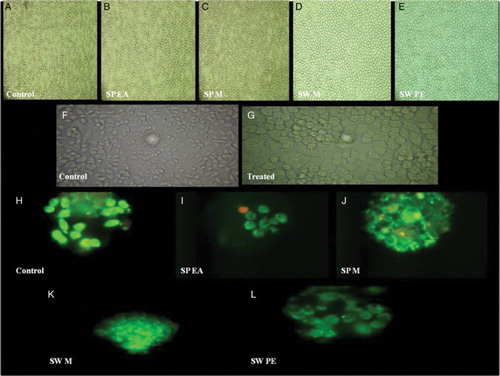
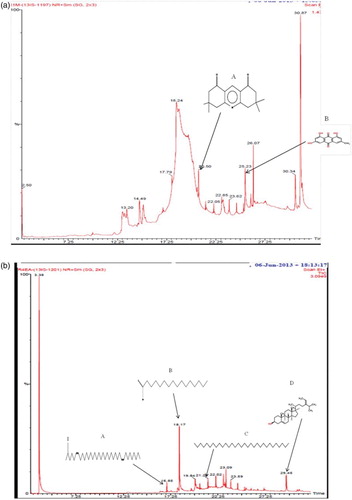
Figure 6. (a) Gas chromatogram of the methanol extract of Sargassum polycystum: A= 3,3,6,6-tetramethyl-1,2,3,4,5,6,7,8-octahydro-1,8-acridinedione; B= 9,10-anthracenedione, 1,3,8-trihydroxy-6-methyl. (b) Gas chromatogram of the ethyl acetate extract of Sargassum wightii: A= Z,Z-6,28 heptatriactontadien-2-one; B= n-hexadecanoic acid; C= hentriacontane; D= fucosterol.