Figures & data
Figure 1. Outline of the study procedure. Visit 1, screening, informed consent, and study ID assignment; Visit 2, assessment of study eligibility, preliminary registration, and start of placebo treatment; and Visit 3, reconfirmation of study eligibility, enrollment, and start of study medication.
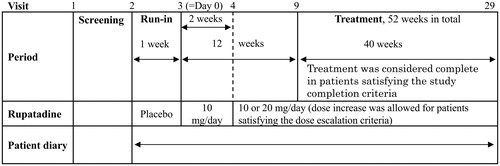
Figure 2. Disposition of study patients. SAS indicates safety analysis set; and FAS, full analysis set.
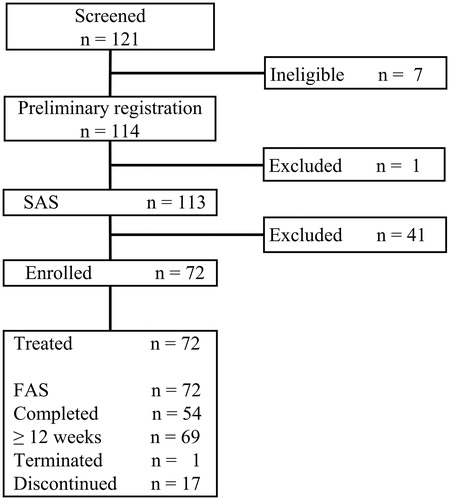
Table 1. Post-hoc analysis of change in the total 4 nasal symptom score (T4NSS) in patients whose rupatadine dose was increased from 10 to 20 mg (Updosed group) and patients whose rupatadine dose remained at 10 mg (10 mg group).
Table 2. Change over time in the JRQLQ score: full analysis set.
Table 3. Treatment-emergent adverse events and adverse drug reactions reported in the treatment period.
Table 4. Treatment-emergent adverse events and adverse drug reactions by duration of treatment and dose in 72 patients treated with rupatadine.
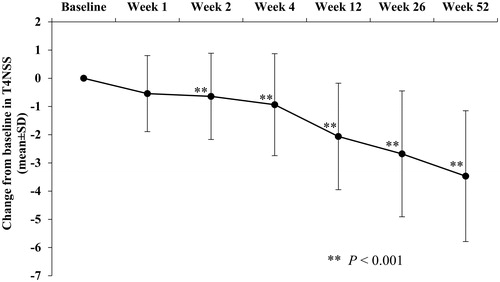
Figure 4. Change over time in T4NSS: full analysis set. The upper and lower bars represent the SE. T4NSS indicates total 4 nasal symptom score; and SE, standard error.
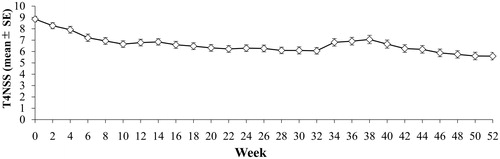
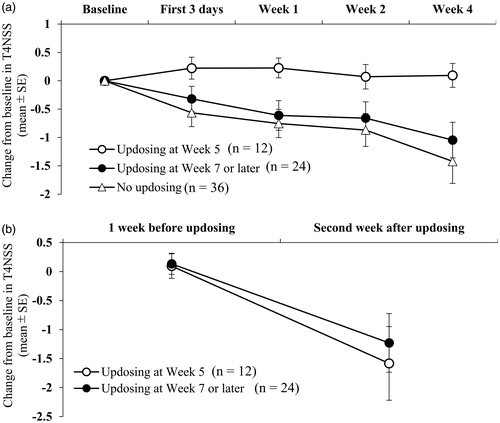
Figure 5. Post-hoc analyses of change from baseline in total 4 nasal symptom score (T4NSS) by the timing of rupatadine updosing to 20 mg: updosing at week 5, updosing at week 7 or later, and no updosing. (a) The changes over the first 4 weeks of treatment. Patients who had no updosing and patients who underwent updosing at Week 7 or later showed the tendency of better improvement at Week 1 (p = 0.033 and p = 0.040, respectively) and Week 4 (p = 0.033 and p = 0.021, respectively) than patients who underwent updosing at Week 5. (b) The changes from 1 week before updosing to the second week after updosing.