Figures & data
Figure 1. Synopsis of the study design. After a screening visit all eligible volunteers will attend V2 in which they will have pre-dose blood tests and vascular studies, and then will be randomized to either single sc dose of alirocumab 150 mg or placebo. They will then attend V3 14 ± 3 days later to have pre-dose blood tests and vascular studies and be administered another sc dose of alirocumab 150 mg or placebo. After this all participants in both groups (alirocumab and placebo group) will be prescribed atorvastatin 20 mg once nightly for 14 days. They will then attend V4 for post-dose blood tests and vascular studies. A follow up visit (V5) will take place 7–14 days later. Abbreviations. SC, subcutaneously; V1, Visit 1; V2, Visit 2; V3, Visit 3; V4, Visit 4; V5, Visit 5.
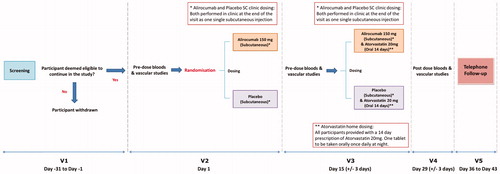
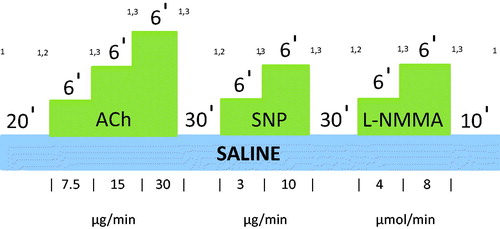
Figure 2. Ach, SNP and L-NMMA infusion pattern in FBF studies. FBF will be measured at baseline and after 20 mins of saline intra-arterial infusion at a rate 1 ml/min. Following this, 3 different concentrations of Ach will be infused intra-arterially at the same rate for 6 mins each. At the last 3 mins of each infusion period FBF will be measured. After 30 mins of washout 2 different concentrations of SNP will be infused intra-arterially for 6 mins each, and FBF will be measured again at the last 3 mins of each infusion period. Following 30 mins washout period 2 different concentrations of L-NMMA will be infused intra-arterially for 6 mins each, and FBF will be measured again at the last 3 mins of each infusion period. A final 10 min of saline will then be infused. BP and heart rate will be recorded at baseline, and at the end of each infusion. 1Blood pressure and heart rate recorded at baseline and at the end of each infusion. 2Basal FBF recorded after 20-min Saline infusion. 3Forearm blood flow recorded after each dose of challenge agent for 3 min. Abbreviations. Ach, acetylcholine; SNP, sodium nitroprusside; L-NMMA, L-NG-monomethyl-arginine acetate; FBF, forearm blood flow.