Figures & data
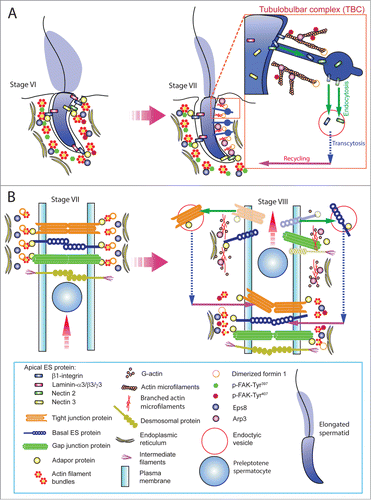
Figure 1. A schematic drawing that illustrates the morphological features of the ectoplasmic specialization (ES) and its relative location in the seminiferous epithelium of adult rat testes. The left panel depicts a stage VII tubule in which the BTB physically divides the seminiferous epithelium into the basal and the adluminal (apical) compartment. In the adluminal compartment, formin 1 is predominantly expressed at the concave (ventral) side of spermatid head but only at stage VII to be used for actin nucleation at the barbed end of an existing microfilament. This is likely being used to facilitate remodeling of the site to allow endocytic vescile-mediated protein trafficking events (see right panel) that begins at late stage VII through VIII of the epithelial cycle, involving protein endocytosis, transcytosis and recycling. At the basal compartment, preleptotene spermatocytes transformed from type B spermatogonia are being transported across the BTB that begin at late stage VII so that formin 1 is also involved in remodeling of the BTB since formin 1 remains considerably expressed at the BTB until late stage VII. Formin 1 is likely working in concert with branched actin polymerization proteins, such as the Arp2/3 complex, to facilitate BTB remodeling.
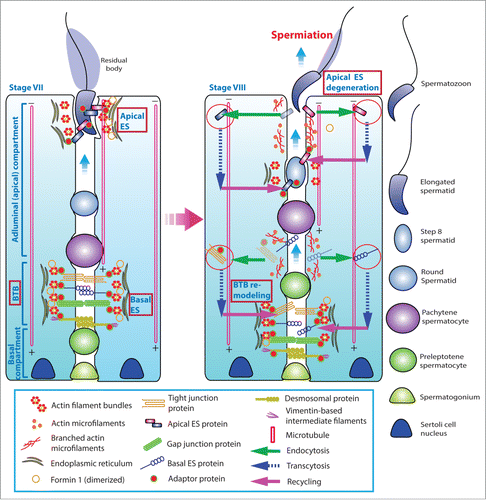
Figure 2. Functional domains of members of formin family. (A) The functional domains of members of the formin family (upper panel). Formins are activated by Rho GTPase following its binding onto the GBD domain, which can also be auto-inhibited by DAD via its action onto DID domain. The functional domains of formin 1 are also shown (lower panel). (B) Two formin polypeptide chains are recruited together at their CC and DD domains, and dimerized via their FH2 domains by creating a functional formin molecule which is capable of nucleating actin microfilament at its barbed end/+ (plus) end by adding actin monomers using the G-actin/profilin complexes. This thus promotes actin polymerization, forming long stretches of actin microfilaments rapidly, necessary to create bundles of actin microfilaments at the ES. Abbreviations used: CC, coiled-coil domain; CID, catenin interacting domain; DAD, diamphanous autoregulatory domain; DD, dimerization domain; DID, diaphanous inhibitory domain; FH, formin homology; FSI, Formin Spir interaction motif; GBD, GTPase-binding domain; MTB, microtubule binding domain.
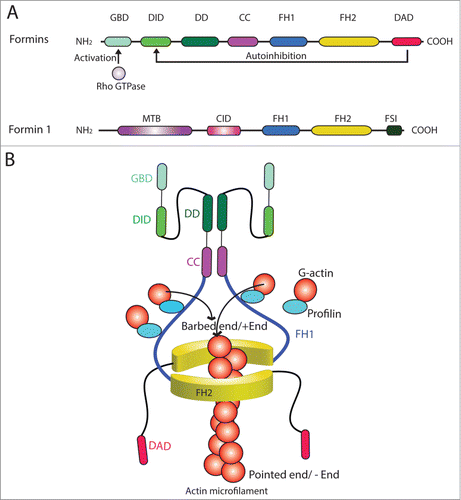
Table 1. Functions of formins in mammalian cells
Figure 3. For figure legend, see next page. Figure 3 (See previous page). A hypothetical model that illustrates the involvement of formin 1 in regulating actin microfilament organization at the ES. (A) At the apical ES (left panel), formin 1 is not expressed in any stage of the epithelial cycle such as at stage VI in which bundles of actin microfilaments serve as the attachment sites for adhesion protein complexes, such as laminina-333/α6β1-integrin and nectin 2/nectin 3 adhesion protein complexes. However, formin 1 is robustly expressed at the concave side of spermatid heads at stage VII (right panel) in an ultrastructure known as the apical TBC, representing giant endocytic vesicles (see enlagred view in the boxed area). This endocytic vesicle-mediated protein trafficking event is facilitated by changes in the organization of actin microfilaments via the concerted efforts of the Arp2/3 complex and formin 1 (note: both Arp3 and formin 1 are highly expressed and localized to the same site in stage VII tubules) which, in turn, facilitate the events of endocytosis, transcytosis and recycling so that apical ES proteins can be recycled to assemble “new” apical ES for the newly derived step 8 spermatids that appear in stage VIII of the cycle. (B) Formin 1 is also robustly expressed at the BTB in stages V-VII, likely being used to maintain the actin microfilaments and to facilitate their organization into a bundled configuration to serve as attachment sites for adhesion protein complexes (left panel). At stage VIII, formin 1 expression at the basal/BTB is considerably diminished, and the Arp3 expression is up-regulated at the site. The intrinsic activity of Arp2/3 complex thus induces branched actin polymerization, converting the microfilament bundles into a branched/unbundled configuration to facilitate the events of endocytosis, transcytosis and recycling so that the “old” BTB adhesion proteins can be recycled to assemble a “new” BTB behind the preleptotene spermatocyte being transported across the immunological barrier (right panel). In short, actin microfilaments at the apical ES and the basal ES/BTB can be rapidly re-organized, through the unique and stage-specific spatiotemporal expression of formin 1, Arp3, and possibly other actin bundling proteins (e.g., Eps8, fascin 1, palladin) known to be expressed at the site.